Zerial Group
The Zerial Group studies the molecular mechanisms of cell and tissue organisation. Our research crosses biological scales and disciplines to decipher protein interactions within the endosomal fusion machinery, define the processes that establish hepatocyte polarity and understand the biophysics of liver tissue formation. Finally, we exploit our knowledge of the delicate interplay between forces, molecules and cells to design innovative strategies for drug delivery.
For more information about Zerial’s research, visit the Lab website.
Can’t find a position for you on our Careers page? Reach out to us anyway! We want to hear your research proposal!
Contact: [email protected]
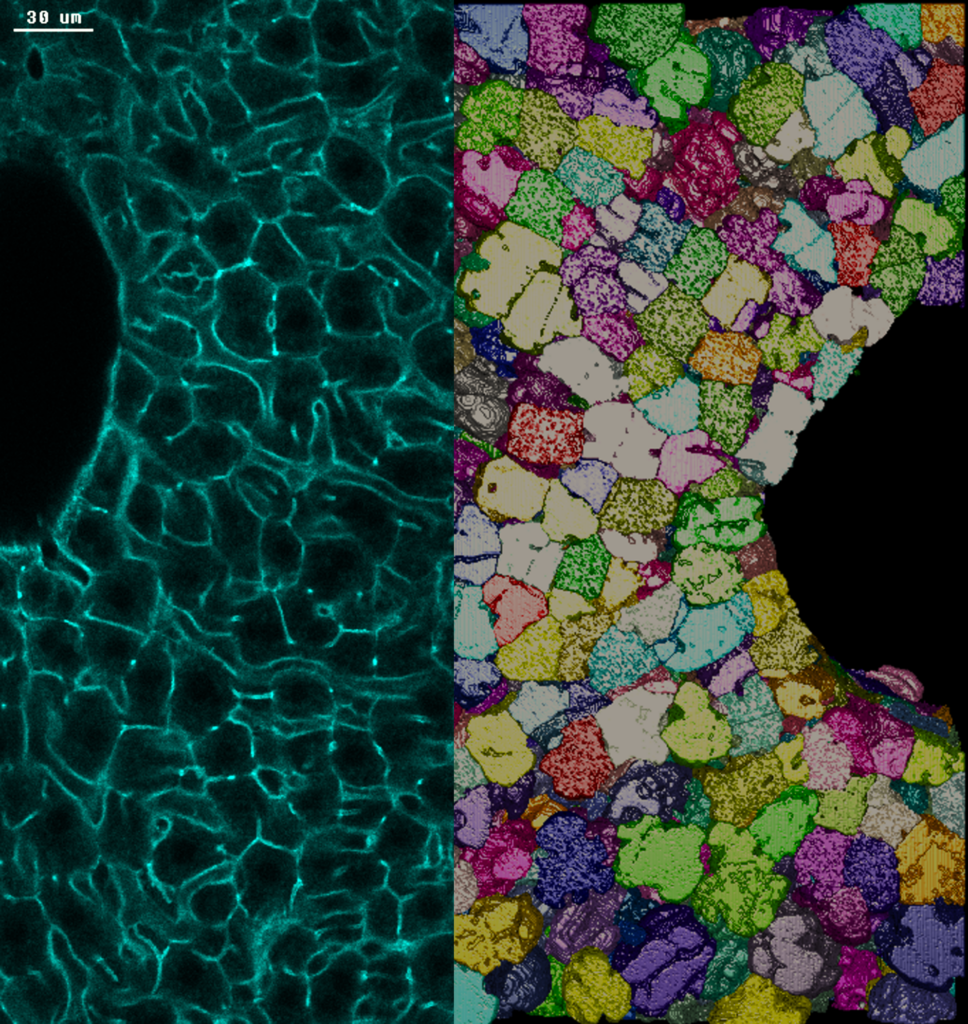
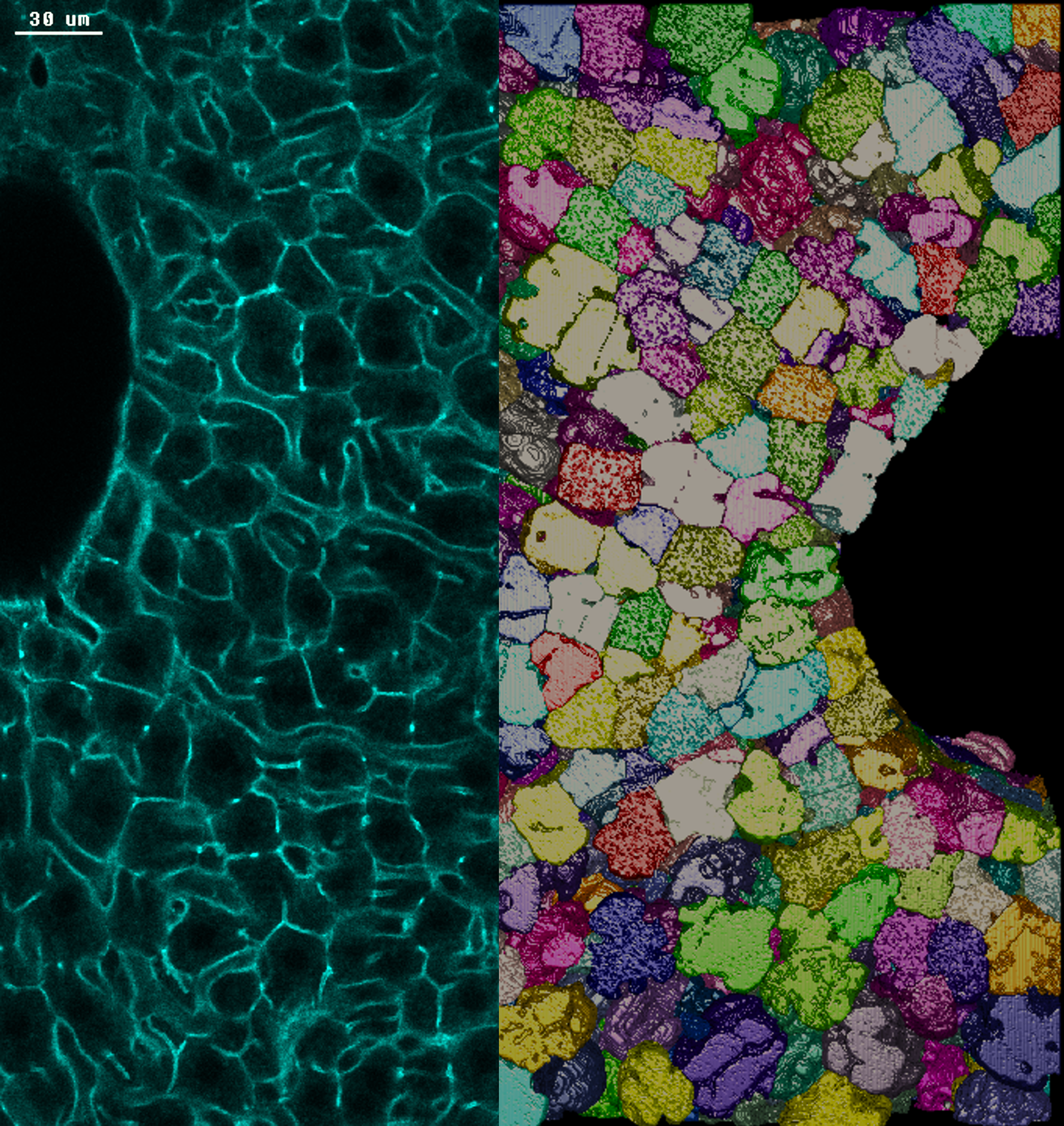
Photo credits: Nuno Pimpao Martins / MPI-CBG. Left: section of adult mouse liver tissue stained with actin. The borders of cells between two large veins are highlighted in green. Right: 3D reconstruction of the borders of hepatocytes, the main metabolic cells of the liver.
Group members
-
 Marino Zerial
Marino Zerial
Director -
 Zhansaya Bauyrzhanova
Zhansaya Bauyrzhanova
PhD Student -
 Marta La Bruna
Marta La Bruna
PhD Student -
 Ilaria Raimondi
Ilaria Raimondi
Technician -
 Lidan Shi
Lidan Shi
Postdoc -
 Chiara Ticli
Chiara Ticli
Postgraduate Fellow -
 José Ignacio Valenzuela Iturra
José Ignacio Valenzuela Iturra
Staff Scientist
Publications
-
09/2005 - Cell
Rab Conversion as a Mechanism of Progression from Early to Late Endosomes
The mechanisms of endosome biogenesis and maintenance are largely unknown. The small GTPases Rab5 and Rab7 are key determinants of early and late endosomes, organizing effector proteins into specific membrane subdomains. Whether such Rab machineries are indefinitely maintained on membranes or can disassemble in the course of cargo transport is an open question. Here, we […]
-
08/1999 - Cell
Oligomeric Complexes Link Rab5 Effectors with NSF and Drive Membrane Fusion via Interactions between EEA1 and Syntaxin 13
SNAREs and Rab GTPases cooperate in vesicle transport through a mechanism yet poorly understood. We now demonstrate that the Rab5 effectors EEA1 and Rabaptin-5/Rabex-5 exist on the membrane in high molecular weight oligomers, which also contain NSF. Oligomeric assembly is modulated by the ATPase activity of NSF. Syntaxin 13, the t-SNARE required for endosome fusion, […]
-
02/1999 - Nature
The Rab5 effector EEA1 is a core component of endosome docking
Intracellular membrane docking and fusion requires the interplay between soluble factors and SNAREs. The SNARE hypothesis1 postulates that pairing between a vesicular v-SNARE and a target membrane z-SNARE is the primary molecular interaction underlying the specificity of vesicle targeting as well as lipid bilayer fusion. This proposal is supported by recent studies using a minimal artificial […]
-
09/1992 - Cell
The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway
We have investigated the in vivo functional role of rab5, a small GTPase associated with the plasma membrane and early endosomes. Wild-type rab5 or rab5ile 133, a mutant protein defective in GTP binding, was overexpressed in baby hamster kidney cells. In cells expressing the rab5ile 133 protein, the rate of endocytosis was decreased by 50% […]
-
07/1990 - Cell
Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments
A set of 11 clones encoding putative GTP binding proteins highly homologous to the yeast YPT1SEC4 gene products have been isolated from an MDCK cell cDNA library. We localized three of the corresponding proteins in mammalian cells by using affinity-purified antibodies in immunofluorescence and immunoelectron microscopy studies. One, the MDCK homolog of rab2, is associated […]