Kalebic Group
The neocortex is the part of brain involved in a variety of higher cognitive functions, including language. During human brain evolution, the neocortex underwent a dramatic expansion, which is considered to be the basis for the unparalleled cognitive abilities of humans. Cognitive impairments often result from altered size or shape of the neocortex, which in turn are caused by defects in the proliferation of neural stem cells in the early stages of brain development. In the adult brain, the same developmental pathways can be hijacked by cancer cells allowing them to proliferate. However, we still understand very little about the mechanisms that are at the basis of those processes.
The research of the Kalebic Group focuses on the molecular and cell biological mechanisms underlying human neocortical development and its implications for neurodevelopmental disorders and brain cancers. In the context of neurodevelopment, we are studying molecular and cellular characteristics of neural stem cells whose impairment results in an alteration of the neocortical size and shape, which can lead to intellectual disabilities, such as Down syndrome. In the context of brain cancers, we are interested in the molecular and cell biology of glioblastoma stem cells to identify new targets associated with the cancer proliferation and invasiveness.
Our Group takes a multidisciplinary approach across biological scales, combining cutting-edge molecular and genetic techniques, CRISPR/Cas9 genome editing, advanced live imaging and computational tools. We apply these techniques to a variety of model systems, including human primary samples, cerebral organoids and animal models.
For informal enquiries please contact [email protected].
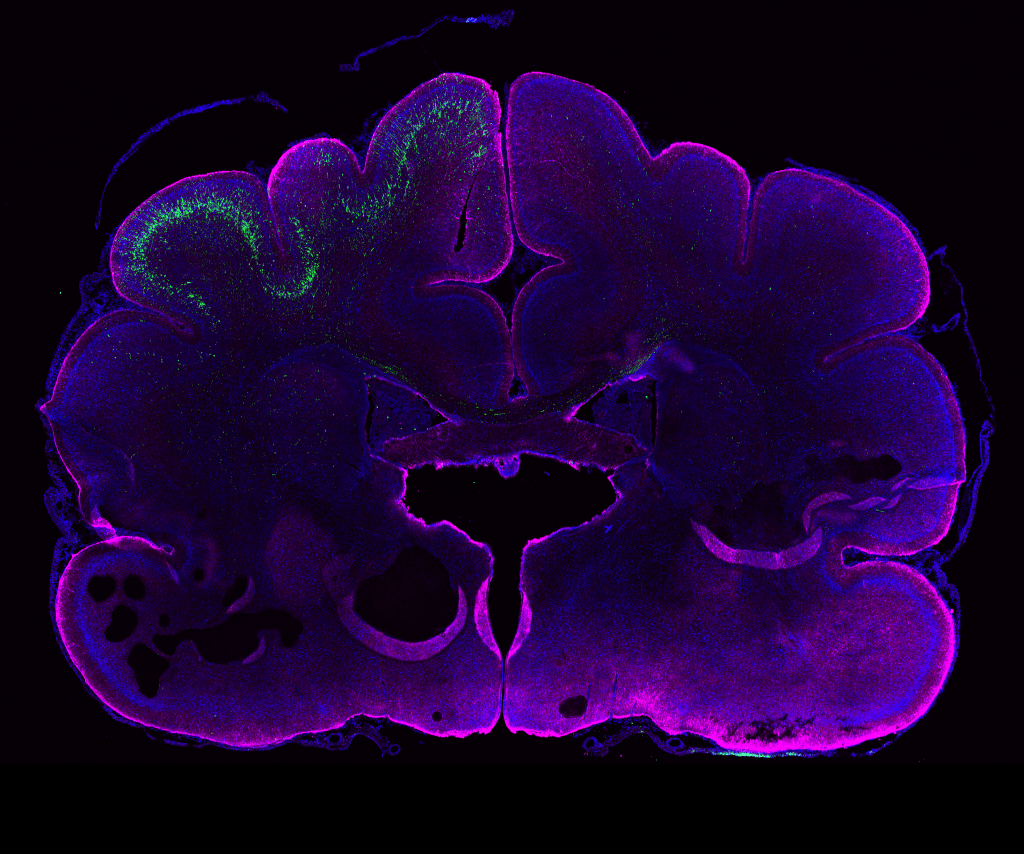
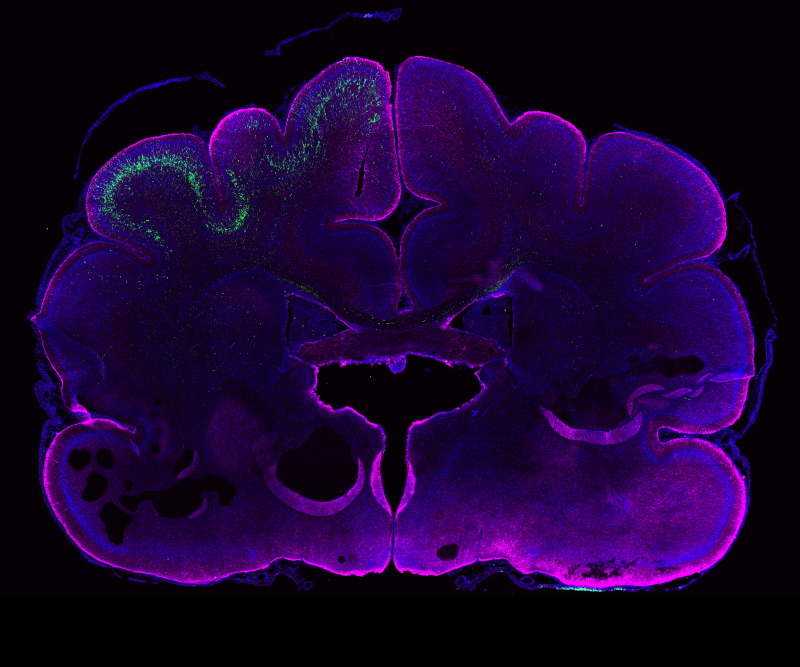
PHOTO:
Genetic manipulation in the ferret brain (age: postnatal day 16). Neural stem cells in the left hemisphere were electroporated in vivo 24 days earlier. GFP+ neurons (green) have their cell bodies in the electroporated hemisphere and extend their processes to the contra-lateral hemisphere. Magenta, GFAP (glial cell marker); blue, DAPI (nuclei).
Image from: Kalebic et al., eLife 2018;7:e41241.
Group members
-
 Nereo Kalebic
Nereo Kalebic
Research Group Leader -
 Negin Alizadehmohajer
Negin Alizadehmohajer
Postgraduate Fellow -
 Carlotta Barelli
Carlotta Barelli
PhD Student -
 Ilaria Bertani
Ilaria Bertani
Senior Research Technician -
 Alberto Campione
Alberto Campione
PhD Student -
 Emanuele Capra
Emanuele Capra
PhD Student -
 Nikola Cokorac
Nikola Cokorac
PhD Student -
 Stefania Faletti
Stefania Faletti
Postdoc -
 Mewhanti Flaminia Kaluthantrige Don
Mewhanti Flaminia Kaluthantrige Don
Postdoc -
 Michela Passi
Michela Passi
Postgraduate Fellow -
 Lucas Van Endert
Lucas Van Endert
Postgraduate Fellow
Publications
-
11/2024 - Life Science Alliance
Morphoregulatory ADD3 underlies glioblastoma growth and formation of tumor–tumor connections
Glioblastoma is a major unmet clinical need characterized by striking inter- and intra-tumoral heterogeneity and a population of glioblastoma stem cells (GSCs), conferring aggressiveness and therapy resistance. GSCs communicate through a network of tumor–tumor connections (TTCs), including nanotubes and microtubes, promoting tumor progression. However, very little is known about the mechanisms underlying TTC formation and […]
-
09/2024 - Nature Methods
Serialized on-grid lift-in sectioning for tomography (SOLIST) enables a biopsy at the nanoscale
Cryo-focused ion beam milling has substantially advanced our understanding of molecular processes by opening windows into cells. However, applying this technique to complex samples, such as tissues, has presented considerable technical challenges. Here we introduce an innovative adaptation of the cryo-lift-out technique, serialized on-grid lift-in sectioning for tomography (SOLIST), addressing these limitations. SOLIST enhances throughput, […]
-
06/2023 - EMBO Reports
DOT1L activity affects neural stem cell division mode and reduces differentiation and ASNS expression
Cortical neurogenesis depends on the balance between self-renewal and differentiation of apical progenitors (APs). Here, we study the epigenetic control of AP’s division mode by focusing on the enzymatic activity of the histone methyltransferase DOT1L. Combining lineage tracing with single-cell RNA sequencing of clonally related cells, we show at the cellular level that DOT1L inhibition […]
-
09/2022 - Science
Human TKTL1 implies greater neurogenesis in frontal neocortex of modern humans than Neanderthals
INTRODUCTION The evolutionary expansion of the neocortex and the concomitant increase in neuron production are considered to be a basis for the increase in cognitive abilities that occurred during human evolution. Endocast analyses reveal that the endocranial volume of modern humans and Neanderthals was similar, suggesting similar brain and neocortex size. But whether similar neocortex […]
-
06/2022 - Frontiers in Cell and Developmental Biology
Forebrain Organoids to Model the Cell Biology of Basal Radial Glia in Neurodevelopmental Disorders and Brain Evolution
The acquisition of higher intellectual abilities that distinguish humans from their closest relatives correlates greatly with the expansion of the cerebral cortex. This expansion is a consequence of an increase in neuronal cell production driven by the higher proliferative capacity of neural progenitor cells, in particular basal radial glia (bRG). Furthermore, when the proliferation of […]