Thyroid: Understanding the Molecular Mechanism of Thyroxine Synthesis
A study from Human Technopole, the University of Milano-Bicocca and Università di Milano reveals new details about how thyroxine – the primary form of thyroid hormone in the blood – is formed, shedding light on a mechanism involving a specific type of amino acid in the thyroglobulin protein. The findings are published in The Journal of Biological Chemistry and highlighted with the journal’s cover.
Thyroid hormones are produced by the thyroid gland and are essential for regulating metabolism and other vital body functions. Dysregulated synthesis of thyroid hormones leads to thyroid disorders such as hypothyroidism and hyperthyroidism that affect millions of people worldwide. The primary thyroid hormone, thyroxine (T4), is synthesised by a complex process within a large protein called thyroglobulin. The exact process through which T4 is produced has remained poorly understood due to thyroglobulin’s size and complexity. Understanding how T4 is synthesised at the molecular level can have wide-reaching impacts. Learning more about the biochemistry of this process can pave the way to developing new treatments or diagnostic tools for hypothyroidism and hyperthyroidism. Additionally, this knowledge might lead to better ways of producing synthetic thyroxine, which is critical for thyroid hormone replacement therapy.
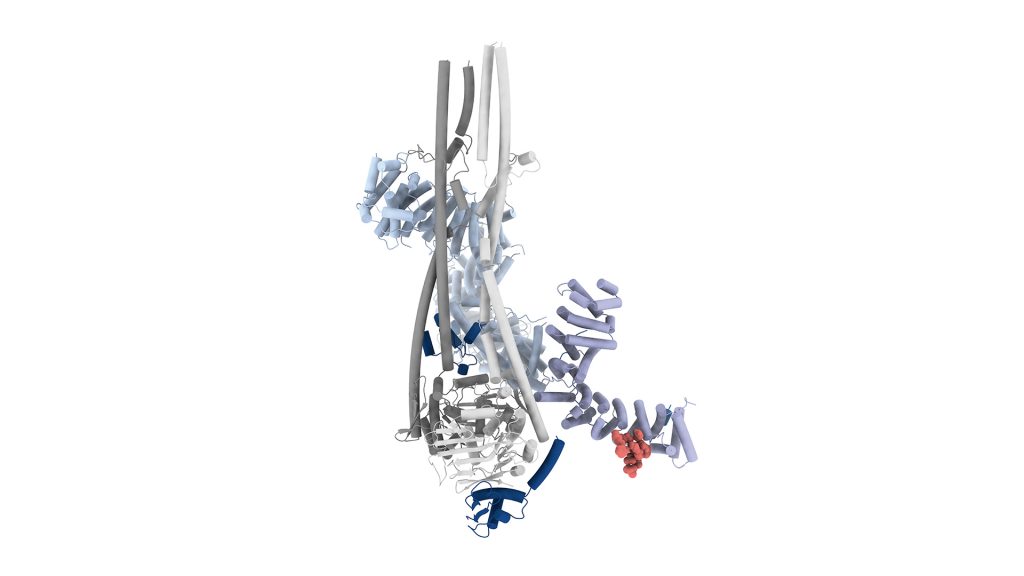
Thyroglobulin has multiple sites where the hormone can form, but only specific tyrosine residues are involved in producing T4. Interestingly, an acidic residue like, for example, glutamate or aspartate always appears next to the active site, but the role of this residue in the synthesis of T4 is unclear.
The groups of Francesca Coscia – Group Leader at Human Technopole – Luca Bertini – Associate Professor at the Università Milano-Bicocca – and Luca Mollica – Associate Professor at the Università di Milano – set out to investigate the function of this residue using a simple protein model, combined with both wet-lab experiments and advanced computer simulations. The researchers discovered that the acidic residue next to the hormonogenic tyrosine plays a key role in facilitating the production of T4 by helping remove a proton from the iodinated tyrosine, which is a crucial first step in the reaction that produces the hormone. Engineered thyroglobulin proteins lacking this acidic residue produced significantly less thyroxine.
These findings showed that thyroxine synthesis is a radical reaction – a highly energetic process triggered by the proximity of an acidic group that stabilises the intermediate steps.
It also demonstrated how the positioning of key amino acids determines the efficiency and direction of hormone production within thyroglobulin. This directional synthesis is crucial because it ensures the hormone can be properly released from the protein at the right time and place.
In summary, the study highlights how small changes in protein structure can profoundly influence hormone production, offering new insights into thyroid biology. It also opens avenues for further research into how other factors, like iodine availability or genetic mutations, might impact thyroid function. Ultimately, this could improve our understanding of thyroid disorders and our ability to treat them effectively.
A conserved acidic residue drives thyroxine synthesis within thyroglobulin and other protein precursors. Stejskalova, Camilla et al. Journal of Biological Chemistry, Volume 301, Issue 1, 108026. DOI: 10.1016/j.jbc.2024.108026