How thyroid hormones are transported into target cells
An international collaboration between Human Technopole and Erasmus Medical Centre researchers sheds light on the molecular mechanisms underlying the transport of thyroid hormones in human cells by monocarboxylate transporters (MCTs). The results of the research are published in Nature Communications.
Thyroid hormones, including thyroxine (T4) and triiodothyronine (T3), are essential for cellular development and metabolic regulation. They are synthesised in the thyroid gland, released into the bloodstream and transported across cell membranes by MCT8 and MCT10, members of the MCT family. Mutations in the SLC16A2 gene, which encodes for MCT8, lead to severe neurodevelopmental and metabolic disorders due to impaired thyroid hormone transport (Allan-Herndon-Dudley syndrome). Hence, understanding the mechanisms of MCT8 and MCT10 transport is crucial for identifying therapeutic targets for MCT8-related conditions.
The group of Francesca Coscia (Human Technopole, Italy) in collaboration with Edward Visser (Erasmus Medical Centre, The Netherlands) used cryogenic electron microscopy (cryo-EM) to resolve five structural states of MCT8 and MCT10, capturing ligand-free, thyroxine-bound, and inhibitor-bound conformations, as well as a patient-derived MCT8 mutant (D424N). Structural data were supported by a plethora of biophysics binding assays and cellular T4 uptake. The obtained structural snapshots provided insight into the transport cycle of T4 and the molecular basis of MCT8 inhibition by silychristin. These structural analyses revealed that T4 binding induces a series of conformational changes in MCT8 and MCT10, involving a network of conserved residues, including R371, Y339, and N119. These residues are strategically positioned to mediate conformational transitions from the outward-facing state (OFS) to the inward-facing state (IFS), facilitating the release of T4 on the opposite side of the membrane.
The structural data show that the ligand-free and T4-bound structures of MCT8 are almost identical, except for slight changes in transmembrane helix 7 (TMH7) that partially occlude the gate. This localised conformational change triggers a cascade of molecular adjustments that facilitate T4 release in the IFS.
The researchers also examined the pathogenic MCT8 D424N mutant, which is associated with severe neurodevelopmental impairments in patients. The D424N mutation introduces a subtle but significant conformational change that disrupts the stability of the central gate, impairing T4 transport despite retaining T4 binding capacity. Structural analysis of the D424N mutant revealed that the mutation increases the flexibility of TMH7 and TMH10, leading to a less efficient closure of the gate and compromised transport activity. This finding suggests that the D424 residue plays a crucial role in maintaining the structural integrity of the transporter during the transition from OFS to IFS.
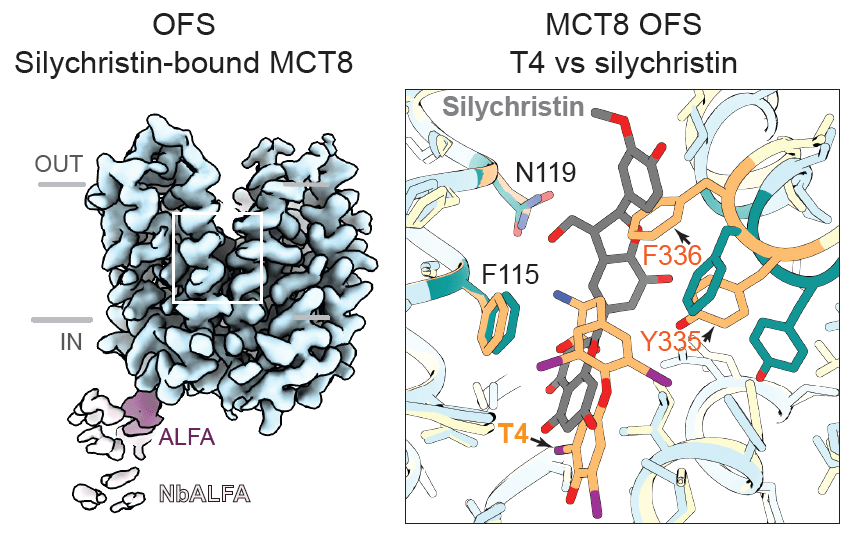
Finally, the teams investigated the inhibitory mechanism of silychristin, a plant-derived compound known to selectively inhibit MCT8. Cryo-EM analysis of MCT8 bound to silychristin revealed that the inhibitor occupies the same binding pocket as T4 but with distinct interactions that prevent the conformational changes required for T4 transport. Silychristin stabilises MCT8 in the outward-facing state, effectively locking the transporter in a non-productive conformation.
This study has significant implications for understanding the molecular basis of thyroid hormone transport and its disruption in disease states. By elucidating the structural dynamics of T4 binding, transport, and inhibition, the researchers provide a comprehensive framework for identifying potential therapeutic targets in MCT8-related disorders. The identification of critical residues involved in T4 transport, such as D424, R371, and F336, suggests potential sites for drug development aimed at stabilising or modulating transporter conformation. Additionally, the differential binding profiles of T4 and silychristin highlight the potential for designing selective inhibitors that can modulate MCT8 activity without affecting MCT10, offering a therapeutic strategy for treating conditions associated with MCT8 dysfunction.
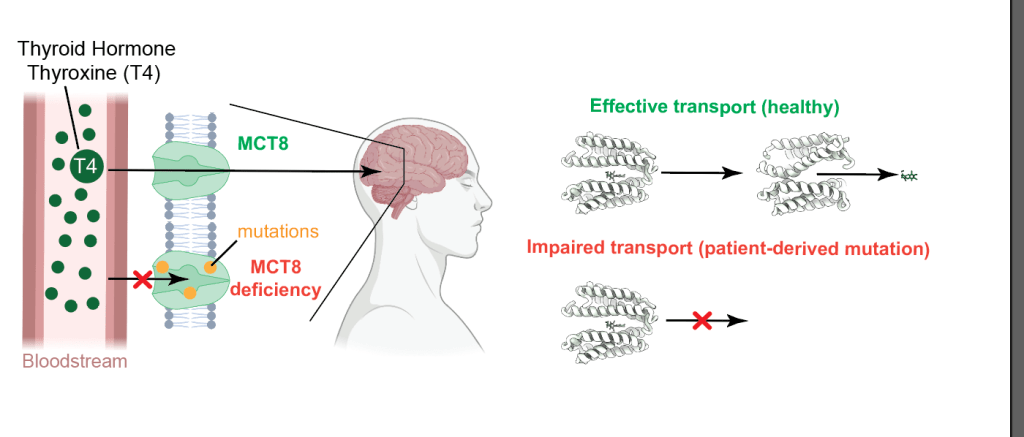
Furthermore, the structural data provide a basis for understanding how subtle conformational changes induced by mutations or inhibitors can dramatically alter transporter function. This insight is particularly relevant for developing therapeutic approaches to restore transporter function in patients with MCT8 deficiency. The use of cryo-EM to resolve multiple states of MCT8 and MCT10 underscores the value of structural biology in uncovering the molecular mechanisms of transport proteins, paving the way for targeted therapeutic interventions in metabolic and neurodevelopmental disorders linked to thyroid hormone transport.
Tassinari, M., Tanzi, G., Maggiore, F. et al. Molecular mechanism of thyroxine transport by monocarboxylate transporters. Nat Commun 16, 4493 (2025). https://doi.org/10.1038/s41467-025-59751-w
This work was funded by ERC starting grant THYROMOL #101041298 to Francesca Coscia.
Images from this article were created using BioRender.