A new model system for cortical development in vitro
Researchers from Human Technopole, the Institute of Molecular Biotechnology and Bicocca University established a method for developing brain assembloids that allows reproducing salient aspects of the antero-posterior polarity of the human cerebral cortex in vitro and opens new possibilities for disease modelling. The study is published in Nature Methods.
The cerebral cortex – the outermost layer of the brain – plays critical roles in memory, thinking, learning, reasoning, and sense. Its development, or corticogenesis, requires the concerted expression of transcription factors and the activation of signalling cascades for different areas and functions to mature properly.
Human Pluripotent Stem Cell (hPSC)-derived brain organoids have been extensively used to study corticogenesis and disease-related alterations. However, these models lack the typical anterior-to-posterior organisation and diversity of cell types observed in vivo.
To address this issue, the groups of Giuseppe Testa – Head of the Neurogenomics at Human Technopole and professor at the University of Milan – Veronica Krenn – Human Technopole Early Career Fellow and Group Leader at University of Milan-Bicocca – and Jürgen Knoblich – Deputy Scientific Director of the Institute of Molecular Biotechnology (IMBA) of the Austrian Academy of Sciences – joined forces to developed a new protocol for engineering cortical organoids that recapitulate salient aspects of the polarized transcriptional and signalling patterns observed in vivo.
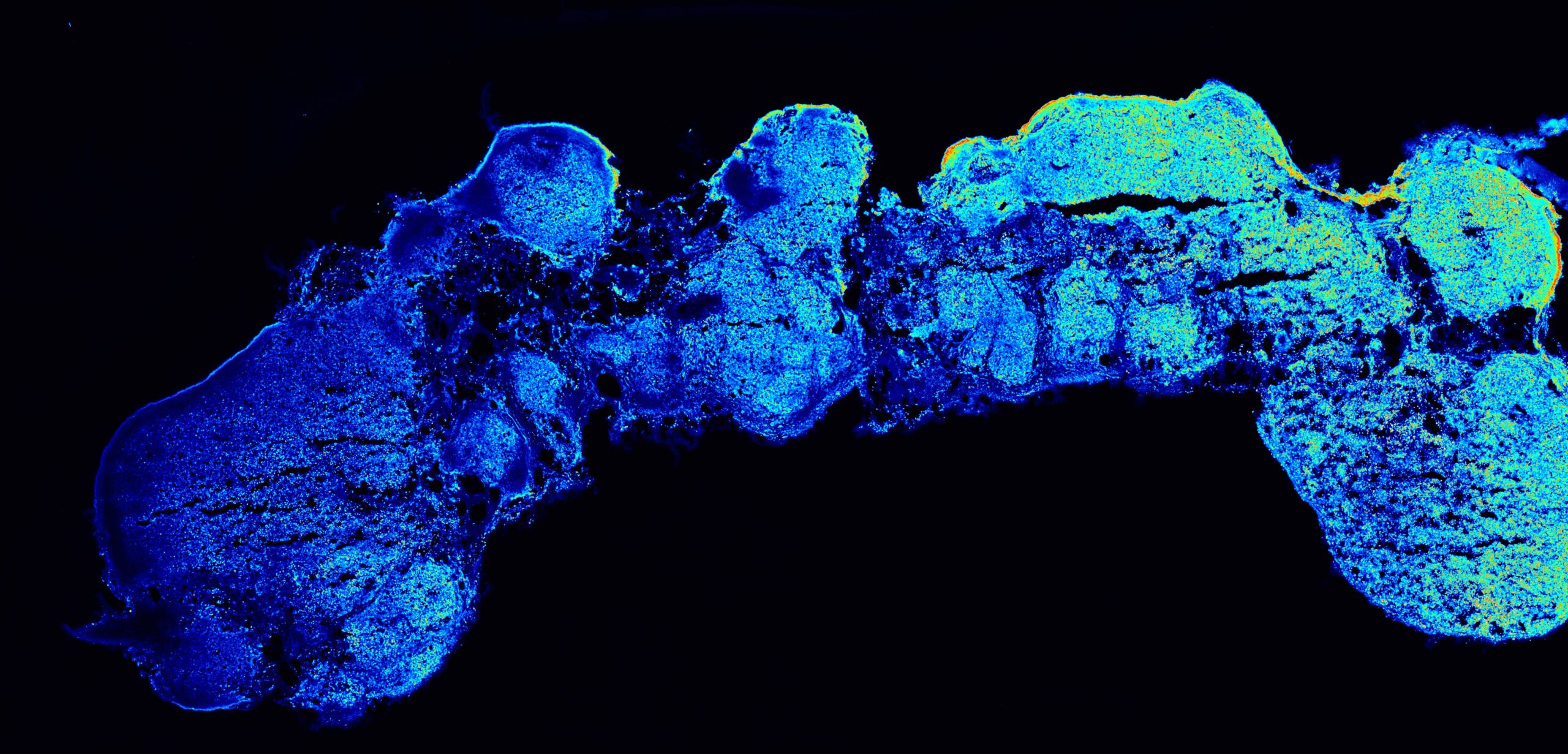
Leveraging the observation that fibroblast growth factor 8 (FGF8) induces a dose-dependent expression of anterior and posterior markers in round cortical organoids, the researchers generated mosaic bodies (mosaic organizer-like embryoid bodies, OrEBs or assembloids) consisting of hPSC-derived brain organoids fused with hPSCs that constitutively produced and secreted FGF8. Such assembloids showed the features of anterior telencephalic development. Based on these findings, the teams increased the length of the organoids in the mosaic bodies to boost the gradient of FGF8 and enable position-dependent effects of FGF8 expression. Strikingly, they observed significant differences in the expression of anterior and posterior markers and fate acquisition at opposite poles of the assembloids. RNA-sequencing analysis on dissected proximal (P), medial (M) and distal (D) segments of individual assembloids confirmed that the P-to-D FGF8 gradient partly induced the in vivo antero-posterior gene expression pattern. Like the developing human brain, each segment showed a different cell-type composition as revealed by single-cell RNA-sequencing. Finally, the researchers focused on Fibroblast growth factor receptor 3 (FGFR3), one of the hits that showed low-to-high P-to-D expression in the assembloids. Mutations in FGFR3 are known to cause achondroplasia, a genetic disorder having as a primary feature dwarfism. Achondroplasia-related mutation in FGFR3 (G380R) decreased the position-dependent proliferation effects along the P-to-D axis of the assembloids.
“Unlike previous attempts that relied on poorly controlled and low-frequency sources of morphogens resulting in local patterning effects in individual organoids, our approach utilises a well-controlled source of FGF8 and culture media formulations with minimal exogenous signals to consistently generate cortical polarity along an organoid’s entire longitudinal axis”, the authors say.
Giuseppe Testa, one of the leaders of this research, also commented, “Polaraised cortical assembloids capture in a dish what had been predicted by the “protomap” model, namely that the diversity of cells from different regions of our cortex is primed early on in development. We are excited about the transformative opportunity that this method now affords to study how genetic and environmental factors contribute to neuropsychiatric disorders by acting on such critical early events”.
In the image: a polarized cortical assembly showing a spatial gradient of expression along its longitudinal axis (from left to right, colors from blue to yellow-red). @Camilla Bosone (IMBA)




