Cancer stem cell morphology predicts glioblastoma growth and resistance
Human Technopole researchers in Milan (Italy) have identified the gene adducin-γ (ADD3) as a crucial regulator of glioblastoma cancer stem cell morphology and intercellular bridges between tumour cells. These connections facilitate communication and allow tumour cells to share resources, evade chemotherapy, and survive challenging conditions. The study has been funded by the AIRC Foundation for Cancer Research, and the findings are published in the journal Life Science Alliance.
Glioblastoma is one of the most aggressive forms of brain cancer, known for its resistance to treatment and ability to spread throughout the brain. A key factor driving this behaviour is the presence of glioblastoma stem cells (GSC), specialised and heterogeneous cancer cells that are highly adaptable and capable of evading therapies. These cells exhibit a remarkable variety of shapes or morphologies, which play a pivotal role in the progression and survival of the disease via the formation of connections between tumour cells (in jargon, tumour cell-tumour cell connections or TTCs).
The group of Nereo Kalebic – Research Group Leader at the Human Technopole – explores an intriguing aspect of glioblastoma biology: how the shape and connectivity of glioblastoma stem cells control cancer growth and resistance to chemotherapy.
In collaboration with the neurosurgery team of Dr. Roberto Stefini at Ospedale Nuovo di Legnano (Italy), the researchers discovered that GSC from patient samples display variable shapes, resembling neural progenitor cells during brain development. This suggests that glioblastoma may hijack developmental programs to drive its own progression.
The team mined public datasets of morpho-regulators in foetal neural progenitor cells to identify genes that might govern the morphology of GSC. By intersecting data in these datasets with a published list of genes expressed in glioblastoma, they identified potential regulators of GSC morphology. In an interdisciplinary collaboration with the group of Francesco Iorio (Human Technopole), the team took advantage of data from the Cancer Dependency Maps and identified adducin-γ (or ADD3) as a critical regulator of glioblastoma stem cell morphology.
The researchers found that when ADD3 was overexpressed in GSC, it led to the formation of more elongated and branched protrusions, strengthening the tumour connectivity network and enhancing tumour resilience. Notably, these changes made the cancer cells significantly more resistant to temozolomide, the primary drug used to treat glioblastoma. On the other hand, reducing ADD3 levels severely impaired the formation of these connections, causing the cancer cells to shrink and eventually die. Carlotta Barelli, the first author of the research and a PhD student in the Kalebic group says “these observations highlight ADD3’s role in determining the aggressive nature of glioblastoma”.
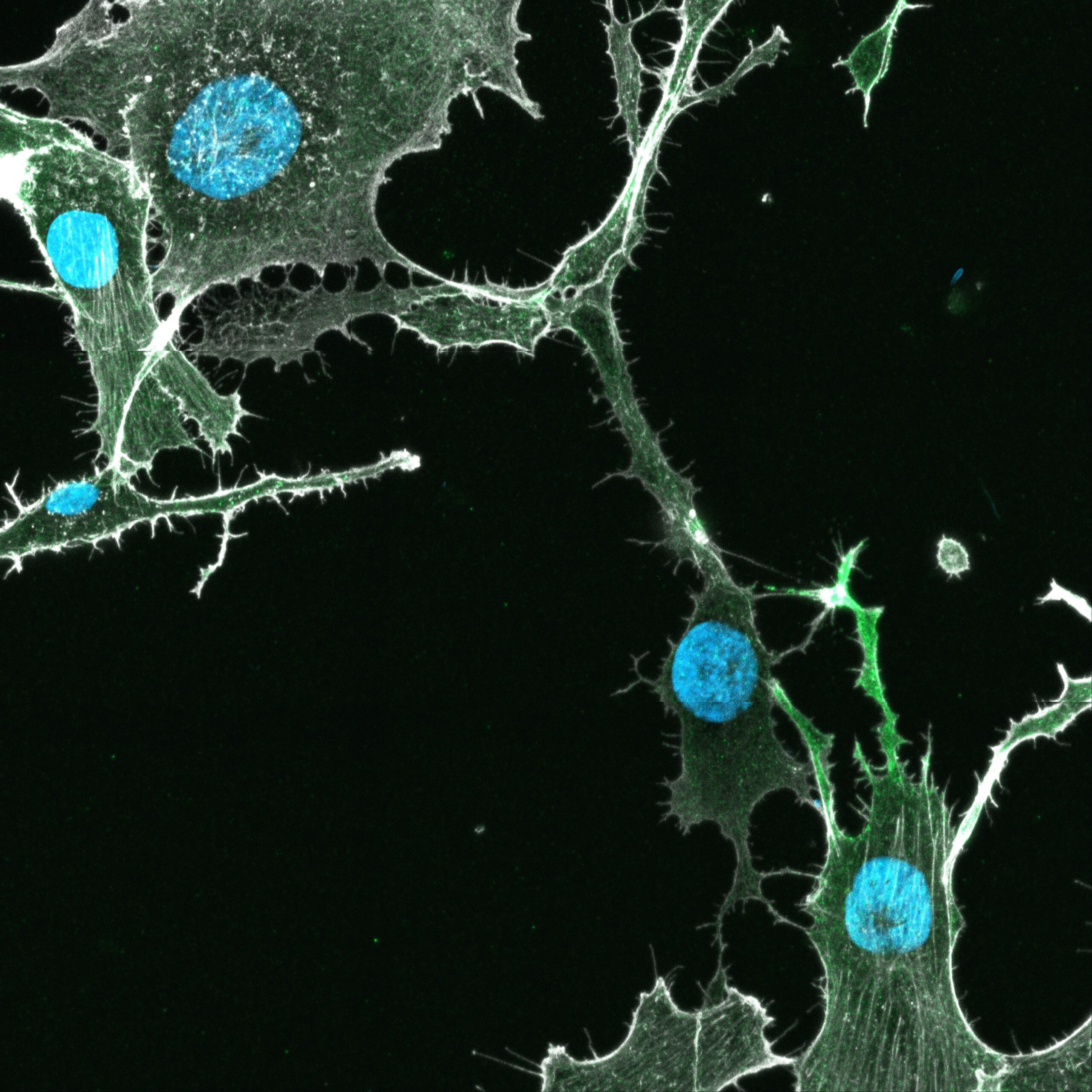
The results also revealed that abrogating ADD3’s ability to stabilise the actin cytoskeleton – the cell’s scaffold – markedly impaired TTC formation. This led to a reduction in cell proliferation and survival, highlighting a potential vulnerability in these otherwise resilient cancer cells.
These findings hold significant promise for improving glioblastoma treatment. By targeting ADD3 or the processes controlled by ADD3, it may be possible to develop new therapeutic strategies that weaken the tumour’s resilience, conferring sensitivity to the existing treatments. Nereo Kalebic, the leader of the study, further explains that “the distinct shapes regulated by ADD3 might serve as biomarkers to identify aggressive tumours or predict how they respond to treatment. Beyond its therapeutic implications, these results highlight a fascinating connection between brain development and cancer, suggesting that insights from neurodevelopmental biology could inform glioblastoma therapy”.
Image: TTCs linking glioblastoma stem cells. Green, ADD3; white, actin; blue; nuclei




