How the human genome condenses during cell division
As a result of an international collaboration, Human Technopole researchers identify M18BP1 as an activator of condensin II through a phosphorylation-regulated competition with MCPH1. This previously unknown mechanism fills a significant gap in our understanding of cell division and suggests avenues for exploring how its misregulation contributes to human disease. The results of the research are published in Molecular Cell.
During cell division, our genetic material undergoes a dramatic transformation: nuclear DNA is compacted and organised into chromosomes that are accurately divided between daughter cells. This process, known as chromosome condensation, is orchestrated by molecular complexes called condensins. There are two types of condensins in human cells – condensin I and condensin II – each with unique roles and regulatory mechanisms. Condensin I and II associate with chromosomes sequentially, with Condensin II and I being required at an early and late phase of DNA condensation, respectively.
A long-standing question in cell biology has been how condensin II is activated and directed to chromosomes specifically during the early stages of cell division (mitosis).
The Group of Alessandro Vannini (Human Technopole, Milan, Italy), in collaboration with researchers at the Max Planck Institute of Molecular Physiology (Dortmund, Germany), The Netherlands Cancer Institute (Amsterdam, the Netherlands) and the University of Sheffield (Sheffield, United Kingdom), addressed this question by identifying MIS18 binding protein 1 (M18BP1) as the critical switch that activates condensin II in dividing mammalian cells.
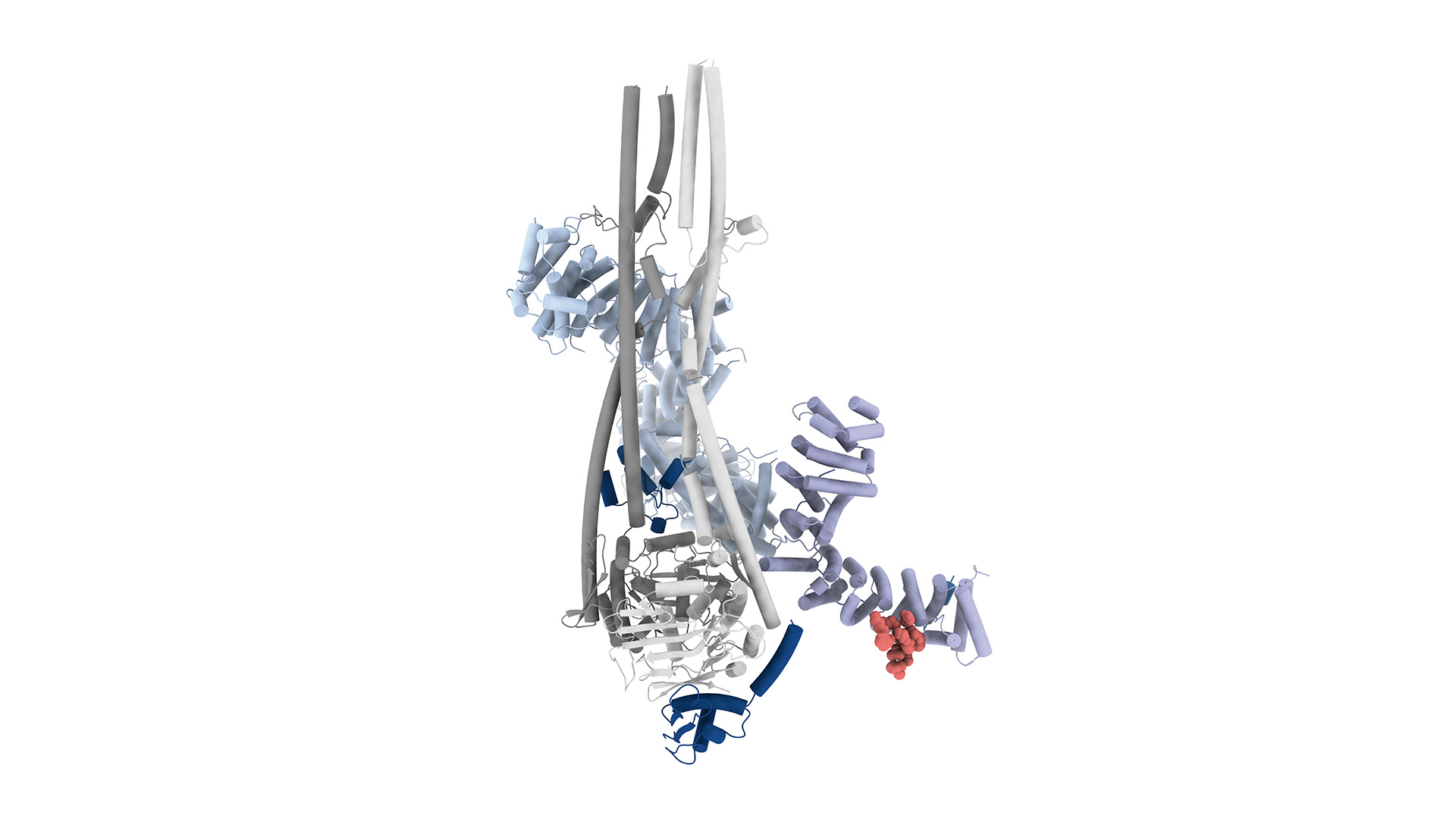
Using genomics and proteomics approaches, the researchers found that M18BP1 binds directly to condensin II through a short segment of its structure, enabling the condensin complex to attach to chromatin (the material chromosomes are made from) and trigger condensation.
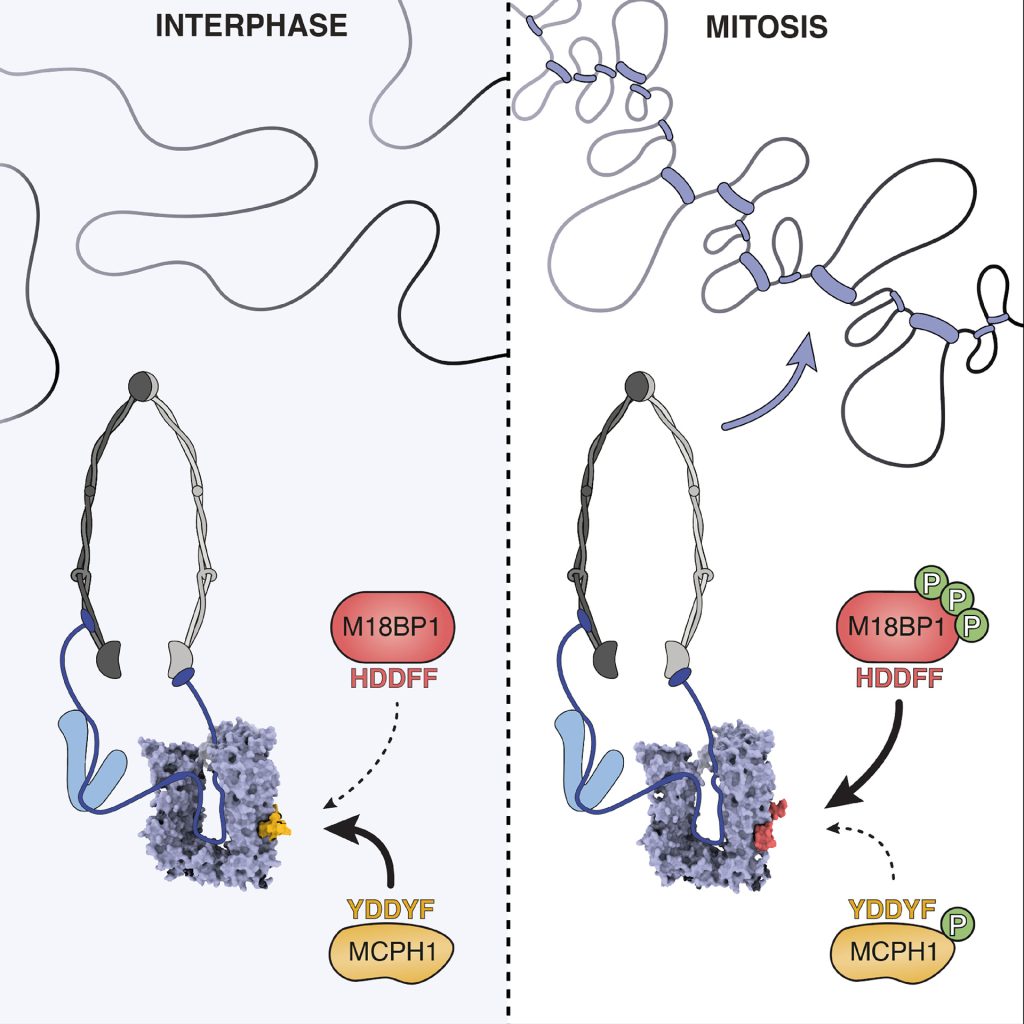
Surprisingly, they found that another protein called MCPH1 blocks this process during most of the cell cycle, by outcompeting M18BP1 for binding to condensin II, thus keeping the genome in a relaxed, uncondensed state until the cell is ready to divide
This direct competition ensures that condensation only begins when appropriate. At the onset of mitosis, the enzyme CDK1 phosphorylates both MCPH1 and M18BP1. This phosphorylation weakens MCPH1’s grip while strengthening M18BP1’s, allowing condensin II to finally access the chromosomes.
Using high-resolution imaging techniques (cryo-EM), the researchers captured how M18BP1 interacts with condensin II, providing a structural map of the complex. They created mutant versions of M18BP1 that couldn’t bind condensin II and showed that without this interaction, chromosome condensation fails, even though other functions of M18BP1(such as helping establish centromeres) remain intact. This confirms a dual role for M18BP1, acting in both chromosome maintenance and mitotic preparation.
These findings not only enhance our understanding of cell division but may also have clinical implications. For example, mutations in MCPH1 are linked to microcephaly, a condition characterised by a smaller brain size. The study suggests that misregulation of condensin II via M18BP1 could contribute to such developmental disorders by causing premature chromosome condensation.