The “tubulin code” in control of ciliary beating
Using advanced cryo-electron microscopy techniques and mutational analysis, HT researchers show that protofilament-specific patterns of tubulin polyglutamylation and glycylation promote the direct binding of axonemal proteins to the ciliary axoneme, thus modulating ciliary beating. The findings are published in Current Biology.
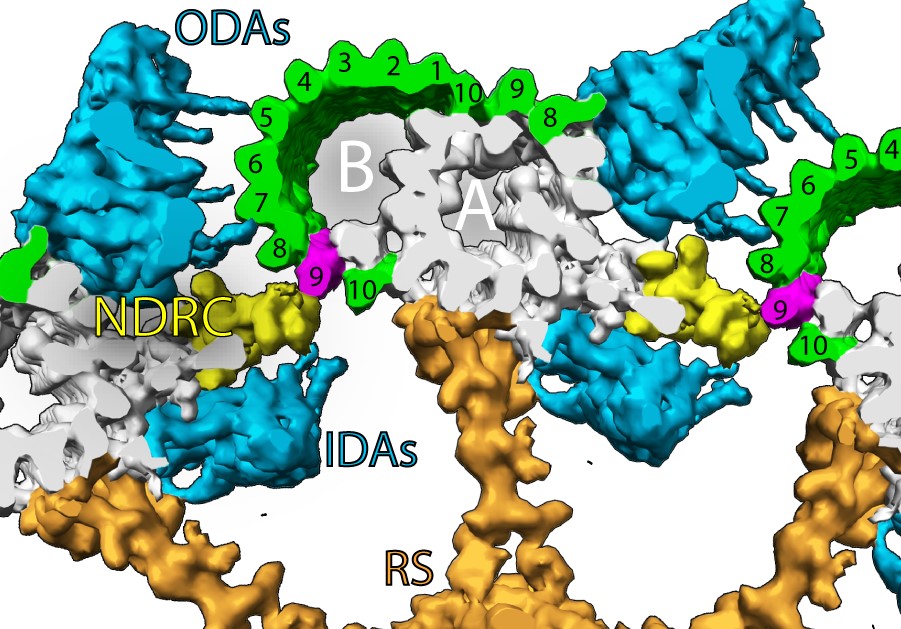
Cilia are microtubule-based mesostructures that control cell motility and signalling in eukaryotic cells. In motile cilia, the core of cilia – or axoneme – consists of microtubules with a “9+2” arrangement in which nine doublet microtubules surround a central pair of singlet microtubules. Axonemal dyneins associate with the outer doublets and induce the microtubules within a doublet to slide onto each other, enabling bending motions. Tubulin posttranslational modifications (PTMs), such as glycylation, glutamylation, acetylation, tyrosination and detyrosination, are known to be enriched in cilia, however their precise molecular distribution and the mechanisms in which they are involved remained elusive. The Pigino Group at Human Technopole previously demonstrated that tubulin glycylation is required for proper ciliary beating in mouse sperm and contributes to the regulation of axonemal dynein activity1. However, unanswered questions remain: do axonemal dyneins directly bind glycylated protofilaments? Are other tubulin PTMs – alone or in combination – playing a role in the binding of axonemal protein complexes to a specific protofilament?
The Pigino Group addressed these issues by leveraging their extensive expertise in immuno-cryo-electron tomography, expansion microscopy, and mutational analysis. Using Chlamydomonas reinhardtii and mice as a model system, the researchers showed that tubulin glycylation and polyglutamylation form mutually exclusive sub-microtubular protofilament-specific nano-patterns in motile cilia. Strikingly, these glycylation and polyglutamylation nanopatterns coincided with the distribution of axonemal dyneins and nexin-dynein regulatory complexes (N-DRCs) on tubulin protofilaments, respectively. In particular, the glycylated protofilaments directly interacted with the microtubule-binding domains of the dynein arms, and depletion of tubulin glycylation impaired Chlamydomonas’ ability to swim. Conversely, tubulin polyglutammylation was important for axonemal integrity and ciliary motility as it allowed the interaction with the N-DRC, which is known to bridge neighbouring microtubule doublets and contributes to the regulation of axonemal dyneins.
“Our results demonstrate that a conserved nanopattern of polyglutamylation and glycylation in the axoneme is required for the proper function of the N-DRC and axonemal dyneins, respectively, thus shedding light on the role of tubulin PTMs in ciliary mechanics”, the authors say.
Gonzalo Alvarez Viar, the first author of the study, also commented “resolving the distribution of two tubulin PTMs at the nanoscale allowed testing our hypothesis of the presence of a nanopatterned ‘tubulin code’, consisting of a protofilament-specific nanopattern of tubulin PTMs, that would reflect the axonemal architecture. This provides a framework to mechanistically understand the specific role of different tubulin PTMs in the regulation of ciliary components that are required for proper ciliary beating”.
1 Gadadhar S. et al. Tubulin glycylation controls axonemal dynein activity, flagellar beat, and male fertility. Science (2021)