Epigenetics and brain development: a matter of metabolism
Neurogenesis and brain development require a tightly regulated balance between symmetric and asymmetric division of neural stem and progenitor cells. How such balance is maintained is unclear: the Taverna and Kalebic Groups now show that the activity of the chromatin modifier DOT1L controls brain neurogenesis by modulating asparagine metabolism in apical progenitor cells.
The cerebral cortex makes up more than half of the volume of the human brain and is responsible for several fundamental functions including reasoning, language, and memory. During embryonic development, two cell types – the neural stem and the progenitor cells – give rise to the neurons within the cerebral cortex. Depending on their localisation, the progenitor cells are classified into apical (APs) and basal progenitors (BP). APs divide asymmetrically generating one AP and one BP, whereas BPs divide symmetrically originating two neurons. Perturbing progenitor cell division results in reduced brain size and neurodevelopmental disorders such as primary microcephaly or “small head syndrome”.
DNA epigenetic changes – reversible modifications of DNA that modify how it is organised and read into cells but not its sequence – were previously found to regulate the balance of AP asymmetric versus symmetric division. However, how epigenetics controls the mode of AP division and drives the onset of human neurodevelopmental disorders is poorly understood.
Elena Taverna and Nereo Kalebic, Group Leaders in the Neurogenomics Research Centre at Human Technopole, in collaboration with Tanja Vogel from Albert-Ludwigs-University in Freiburg (Germany), addressed these questions by using advanced cell lineage tracing, sequencing, and imaging techniques in cell culture and in mice. The results of the research are now published in EMBO Reports.
The teams focused on the chromatin modifier DOT1L, which the Vogel Group had previously identified as a regulator of cortical development in mice. They found that chemical inhibition of DOT1L’s enzymatic activity, namely its ability to reversibly modify the DNA, or downregulation of DOT1L expression enhanced the symmetric division of AP cells and promoted their differentiation into neurons. To investigate how DOT1L activity regulated AP division and differentiation, the researchers looked at the expression of genes in AP and BP cells treated with Pinometostat, a well-known DOT1L inhibitor used to treat cancer and in cell reprogramming. DOT1L inhibition modulated the expression of several genes involved in the regulation of neuronal cell metabolism. In particular, they found that reduced DOT1L activity caused a drop in the activity of chromatin-modifier EZH2, which in turn increased the expression of Asparagine Synthetase (ASNS), an enzyme catalysing the synthesis of amino acid asparagine. Concomitant inhibition of ASNS and DOT1L activities leads to decreased AP differentiation, whereas ASNS overexpression in APs increased it, thus showing that DOT1L mediates AP differentiation via ASNS-dependent metabolic regulation.
“To the best of our knowledge, DOT1L is the first transcriptional regulator described altering AP’s division mode in this manner. By identifying Asns, we describe a previously unknown link among metabolism, epigenetics, and APs, and strengthen the concept that metabolism is a crucial regulator of stem and progenitor cells” the authors say.
In summary, the Taverna, Kalebic, and Vogel Groups show that epigenetics modulates the balance between the symmetric and asymmetric division in APs by regulating cell metabolism. These findings advance our understanding of how precursor-cell fate specification affects brain development.
DOI: 10.15252/embr.202256233
https://www.embopress.org/doi/10.15252/embr.202256233
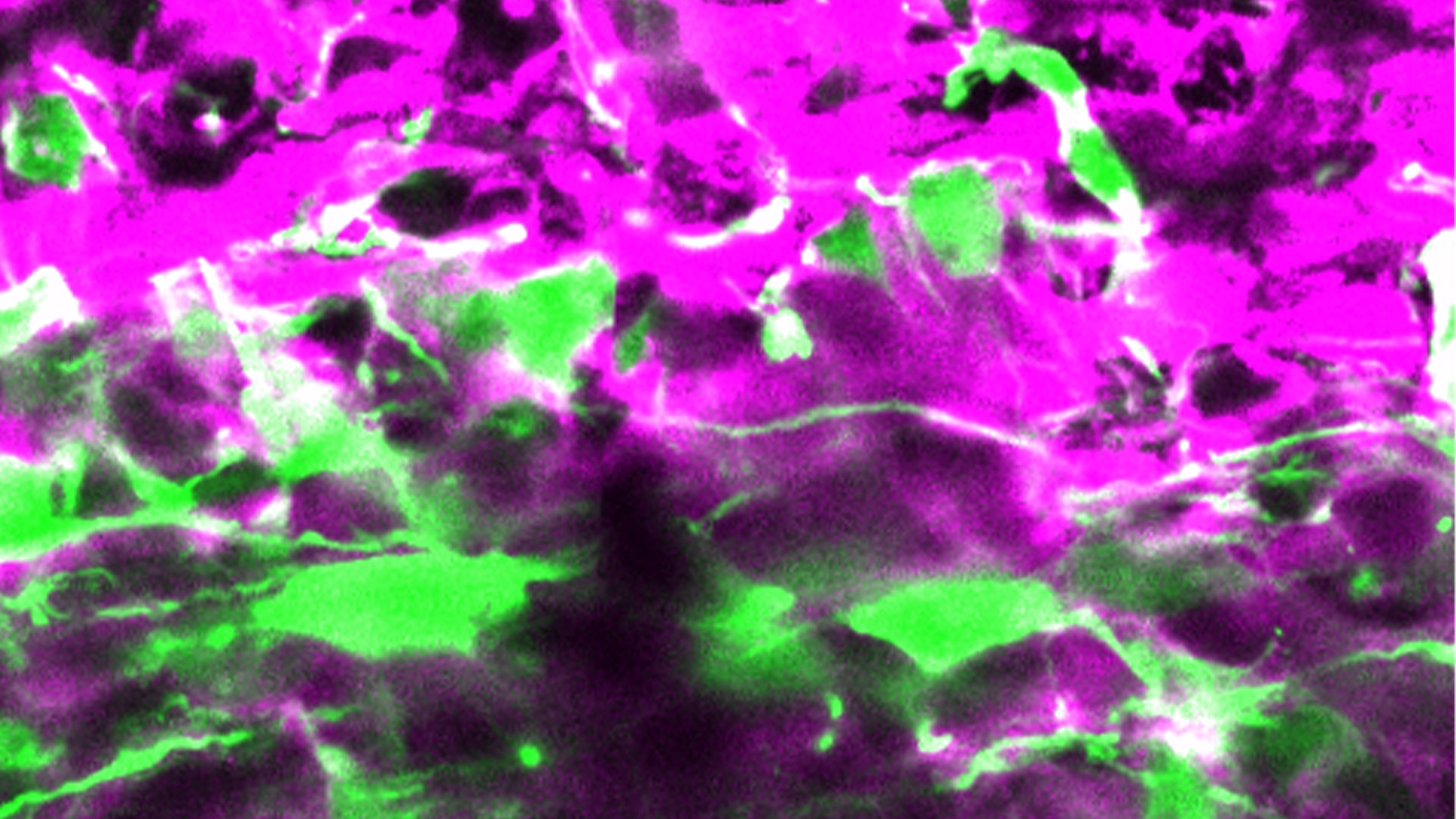
In the image: Knocking down DOT1L in apical precursor cells (green cells) changes the way they divide, favouring the generation of neurons (magenta cells) at the expenses of other stem cell types in developing mouse brain. Image by Chiara Ossola and Elena Restelli.




