Understanding the developmental origins of autism using brain organoids
Chromatin remodelling factor CHD8 is frequently mutated in autism spectrum disorder. However, little is known about how and when CHD8 impacts on brain development. A new study by Giuseppe Testa et al. describes for the first time the effects of CHD8 haploinsufficiency on human neurodevelopment at single cell resolution.
Autism Spectrum Disorder (ASD) is a neurological disorder that induces anxiety, repetitive behaviour, and difficulties in communicating and interacting with other people. Genes coding for chromatin remodelling factors, such as chromodomain helicases, are often mutated in ASD patients, with chromodomain helicase DNA-binding 8 (CHD8) being the most frequently mutated and penetrant among them. CHD8 heterozygous loss-of-function (LoF) mutations lead to an overly large head (i.e. macrocephaly) and ASD-like phenotypes in mammals. While animal models have allowed linking CHD8 to brain development, they have failed to reveal how and at which stage CHD8 affects neurodevelopment. Indeed, CHD8 LoF mutations are usually associated with mild neurological problems or conflicting results in mice. Thus, there is a need for alternative experimental models to understand the developmental origin of ASD in humans.
The team of Giuseppe Testa (Head of the Neurogenomics Research Centre at Human Technopole, Professor of Molecular Biology at the University of Milan, and Group Leader at the European Institute of Oncology) and Gaia Novarino (Principal Investigator at the Institute of Science and Technology Austria – ISTA), used cerebral organoids – miniature organs that closely recapitulate key aspects of the developing human brain in a Petri dish – to investigate the impact of CHD8 haploinsufficiency on human cortical development at single cell resolution. The results of this study are now published in Cell Reports.
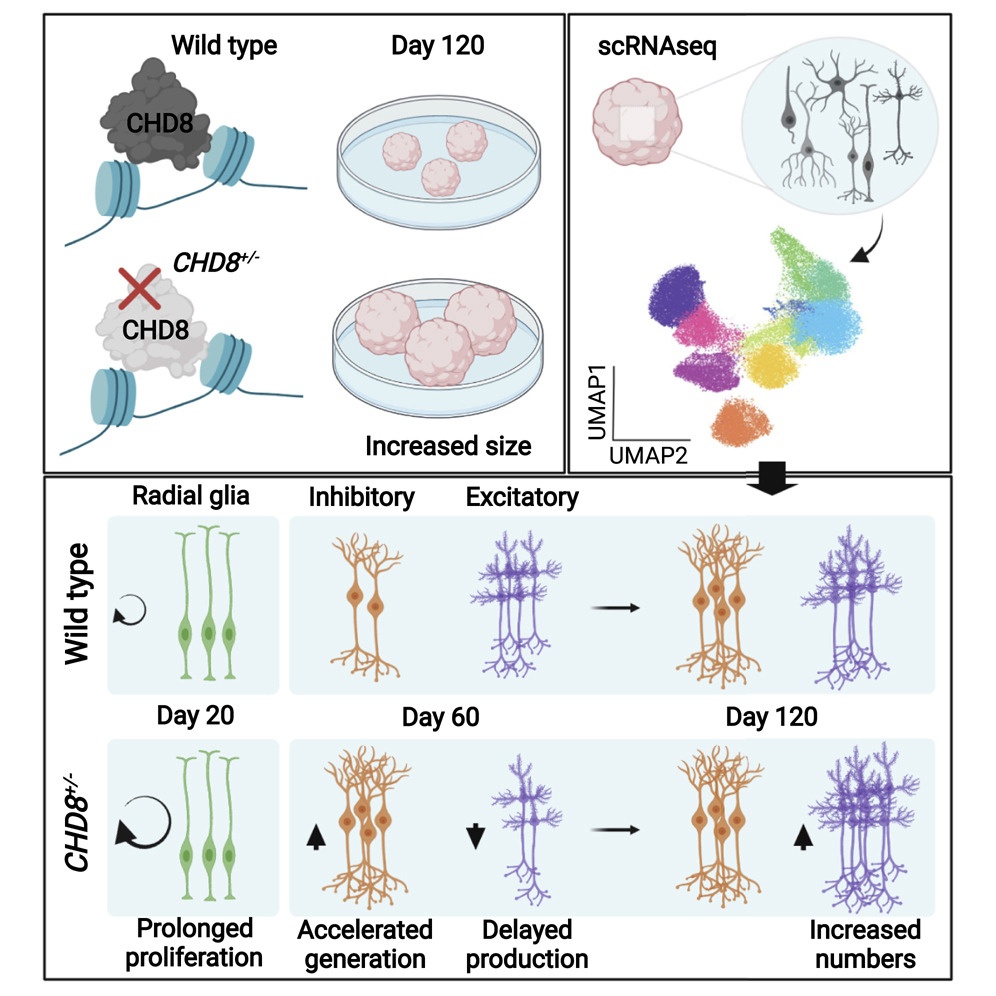
First, the researchers set up a procedure whereby cerebral organoids could reproducibility recapitulate the features of cortical development. Subsequently, they found that CHD8 expression peaked in 36-day old organoids, thus suggesting that CHD8 could specifically regulate that organoid developmental stage. To test this hypothesis, the researchers engineered human Embryonic Stem Cells (hESC), which proliferate unlimitedly and can originate mini organs in vitro, to obtain organoids with a CHD8 copy bearing either an inactivating mutation or two ASD patient-specific mutations, only one of which is associated also with macrocephaly and intellectual disability.
“Finding in brain organoids specific readouts that distinguish the specific mutations observed in patients, for example an increase in organoid size occurring only for mutations of patients with macrocephaly, demonstrates the power of this technology in resolving the complexity of human diseases through intermediate proxies that can be studied in vitro” says Giuseppe Testa.
But how does having only one half of the normal dosage of the CHD8 gene (i.e. having only one functional copy of the two we would normally inherit from our parents) induce such alteration? “To dissect this aspect, we profiled more than 75,000 cells from control and mutant cerebral organoids, revealing that CHD8 haploinsufficiency disrupted the balance between organoid-specific cell populations” says Emanuele Villa, first author of the study. “More in detail, it transiently increased the number of interneurons while decreasing that of excitatory neurons at specific developmental windows. These CHD8 mutations also induced sustained cell proliferation and organoid overgrowth”.
Finally, the teams showed that CHD8 haploinsufficiency affected mRNA processing in post-mitotic neurons altering alternative splicing in a cell-population specific way.
In summary, Villa et al. (10.1016/j.celrep.2022.110615) gleaned from cerebral organoids and single cell transcriptomics insight into the temporal and lineage-specific role of CHD8 in cortical development. While further studies are needed to understand how CHD8 haploinsufficiency mechanistically regulates cell proliferation and differentiation dynamics in the developing brain, these findings pave the way for novel approaches in neurodevelopmental disease modelling.




