Testa Group
The Testa Group harnesses the unprecedented potential of cell reprogramming to study the molecular basis of human neuropsychiatric and neurological diseases (NPD), by chasing the dynamics of their unfolding in physio pathologically relevant models and straddling multiple scales of analysis from single cell resolution to organismal function.
One of the most tangible outputs of somatic cell reprogramming has been a paradigm shift in our ability to model human diseases, for which fundamental limitations have been so far: i) the scarce availability of primary diseased tissues, which is particularly salient for disorders of the nervous system; and ii) the difficulty of reconstructing developmental and patient-specific trajectories during the unfolding of diseases.
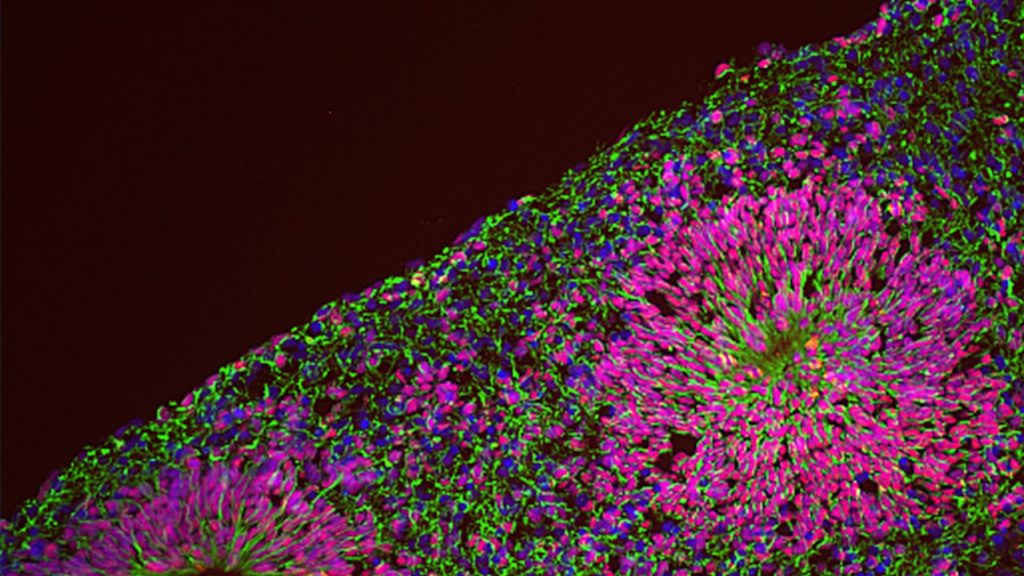
We are thus pursuing NPD modelling with human induced pluripotent stem cells (iPSC) coupled with the differentiation into relevant lineages through a range of complementary experimental paradigms, including glutamatergic neurons by induced expression of neurogenin-2 (NGN2), neural crest stem cells and 3-dimensional brain organoids that recapitulate salient stages of early brain development, including the diversity of cell populations unique to the human brain. This is allowing us to dissect the genetic and environmental components of NPD pathogenesis, by means of several “omics” approaches at a bulk and single cell resolution integrated with high throughput imaging and functional in vitro and in vivo assays.
The Testa Group focuses on a uniquely informative range of syndromes featuring intellectual disability and autism spectrum disorder that are caused by mutations or dosage alterations in epigenetic regulators and transcription factors, including Williams-Beuren Syndrome and 7q11.23 microduplication Syndrome, Kabuki Syndrome, ADNP-related autistic spectrum disorders, Weaver Syndrome, Gabriele-Testa-DeVries Syndrome, as well as on paradigmatic environmental factors that adversely impact on neurodevelopment, namely endocrine disruptive chemicals.
Finally, the prism of human neurodevelopmental disorders is also allowing us to investigate the logic of the gene regulatory networks underlying the evolution of the modern human face and brain, integrating the analysis of craniofacial dysmorphologies and brain alterations to illuminate the evolutionary-developmental trajectories underlying the modern human condition.
Testa Group 2023

Group members
-
 Giuseppe Testa
Giuseppe Testa
Head of Neurogenomics -
 Davide Aprile
Davide Aprile
Postdoc -
 Lorenzo Basile
Lorenzo Basile
Undergraduate Intern -
 Nicolò Caporale
Nicolò Caporale
Postdoctoral Associate -
 Davide Castaldi
Davide Castaldi
Postdoc Fellow -
 Lorenza Culotta
Lorenza Culotta
Senior Technician -
 Francesco Elli
Francesco Elli
Affiliate Scientist -
 Marlene Cristina Faria Pereira
Marlene Cristina Faria Pereira
Affiliate Scientist -
 Samuele Galimi
Samuele Galimi
Postgraduate Fellow -
 Oliviero Leonardi
Oliviero Leonardi
PhD Student -
 Lisa Mainardi
Lisa Mainardi
Scientific Visitor -
 Alessandro Melon
Alessandro Melon
Undergraduate Intern -
 Riccardo Nagni
Riccardo Nagni
Undergraduate Intern -
 Marco Tullio Rigoli
Marco Tullio Rigoli
Affiliate Scientist -
 Ludovico Rizzuti
Ludovico Rizzuti
Affiliate Scientist -
 Adrianos Skaros
Adrianos Skaros
Affiliate Scientist -
 Sarah Stucchi
Sarah Stucchi
PhD Student -
 Alejandro Lopez Tobon
Alejandro Lopez Tobon
Scientific Advisor -
 Alessandro Valente
Alessandro Valente
Affiliate Scientist -
 Alessia Valenti
Alessia Valenti
PhD Student -
 Emanuele Villa
Emanuele Villa
Senior Staff Scientist -
 Alessandro Vitriolo
Alessandro Vitriolo
Affiliate Scientist -
 Narges Yahyazadeh
Narges Yahyazadeh
Scientific Visitor
Publications
-
10/2022 - BioRxiv
7q11.23 CNV alters protein synthesis and REST-mediated neuronal intrinsic excitability
Copy number variations (CNVs) at 7q11.23 cause Williams-Beuren (WBS) and 7q microduplication syndromes (7Dup), two neurodevelopmental disorders with shared and opposite cognitive-behavioral phenotypes. Using patient-derived and isogenic neurons, we integrated transcriptomics, translatomics and proteomics to elucidate the molecular underpinnings of this dosage effect. We found that 7q11.23 CNVs cause opposite alterations in neuronal differentiation and […]
-
10/2022 - BioRxiv
GTF2I dosage regulates neuronal differentiation and social behavior in 7q11.23 neurodevelopmental disorders
Copy number variations at 7q11.23 cause neurodevelopmental disorders with shared and opposite manifestations. Deletion causes Williams-Beuren syndrome (WBS), while duplication causes 7q11.23 microduplication syndrome (7Dup). Converging evidence indicates GTF2I, from the 7q11.23 locus, is a key mediator of the cognitive-behavioral phenotypes associated with WBS and 7Dup. Here we integrate molecular profiling of patient-derived cortical organoids (COs) […]
-
09/2022 - Nature
A nomenclature consensus for nervous system organoids and assembloids
Self-organizing three-dimensional cellular models derived from human pluripotent stem cells or primary tissue have great potential to provide insights into how the human nervous system develops, what makes it unique and how disorders of the nervous system arise, progress and could be treated. Here, to facilitate progress and improve communication with the scientific community and […]
-
05/2022 - Frontiers in Neuroscience
EZH2-Mediated H3K27me3 Targets Transcriptional Circuits of Neuronal Differentiation
The Polycomb Repressive Complex 2 (PRC2) plays important roles in the epigenetic regulation of cellular development and differentiation through H3K27me3-dependent transcriptional repression. Aberrant PRC2 activity has been associated with cancer and neurodevelopmental disorders, particularly with respect to the malfunction of sits catalytic subunit EZH2. Here, we investigated the role of the EZH2-mediated H3K27me3 apposition in […]