Testa Group
The Testa Group harnesses the unprecedented potential of cell reprogramming to study the molecular basis of human neuropsychiatric and neurological diseases (NPD), by chasing the dynamics of their unfolding in physio pathologically relevant models and straddling multiple scales of analysis from single cell resolution to organismal function.
One of the most tangible outputs of somatic cell reprogramming has been a paradigm shift in our ability to model human diseases, for which fundamental limitations have been so far: i) the scarce availability of primary diseased tissues, which is particularly salient for disorders of the nervous system; and ii) the difficulty of reconstructing developmental and patient-specific trajectories during the unfolding of diseases.
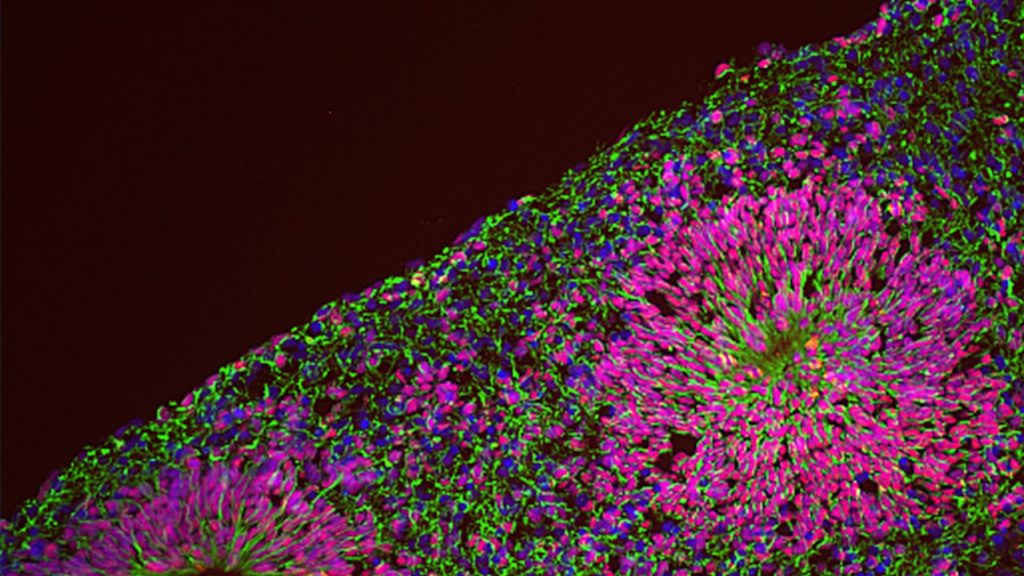
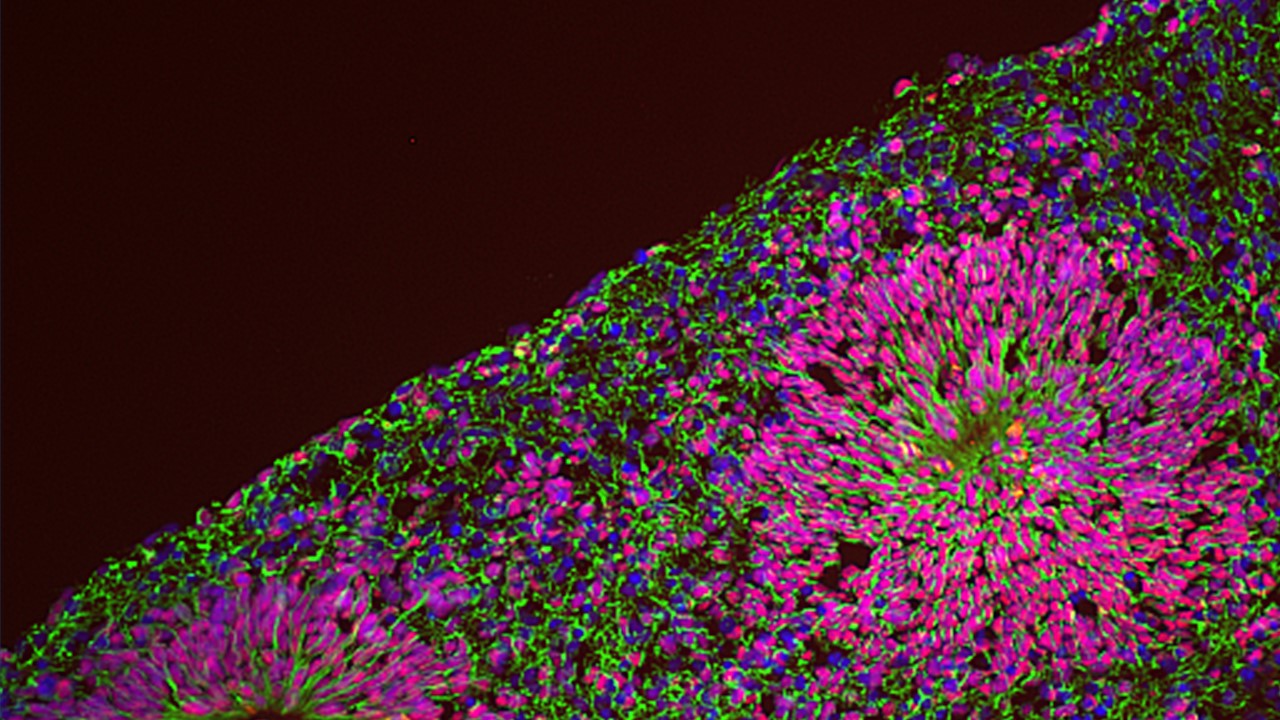
We are thus pursuing NPD modelling with human induced pluripotent stem cells (iPSC) coupled with the differentiation into relevant lineages through a range of complementary experimental paradigms, including glutamatergic neurons by induced expression of neurogenin-2 (NGN2), neural crest stem cells and 3-dimensional brain organoids that recapitulate salient stages of early brain development, including the diversity of cell populations unique to the human brain. This is allowing us to dissect the genetic and environmental components of NPD pathogenesis, by means of several “omics” approaches at a bulk and single cell resolution integrated with high throughput imaging and functional in vitro and in vivo assays.
The Testa Group focuses on a uniquely informative range of syndromes featuring intellectual disability and autism spectrum disorder that are caused by mutations or dosage alterations in epigenetic regulators and transcription factors, including Williams-Beuren Syndrome and 7q11.23 microduplication Syndrome, Kabuki Syndrome, ADNP-related autistic spectrum disorders, Weaver Syndrome, Gabriele-Testa-DeVries Syndrome, as well as on paradigmatic environmental factors that adversely impact on neurodevelopment, namely endocrine disruptive chemicals.
Finally, the prism of human neurodevelopmental disorders is also allowing us to investigate the logic of the gene regulatory networks underlying the evolution of the modern human face and brain, integrating the analysis of craniofacial dysmorphologies and brain alterations to illuminate the evolutionary-developmental trajectories underlying the modern human condition.
Testa Group 2023

Group members
-
 Giuseppe Testa
Giuseppe Testa
Head of Neurogenomics -
 Davide Aprile
Davide Aprile
Postdoc -
 Lorenzo Basile
Lorenzo Basile
Undergraduate Intern -
 Nicolò Caporale
Nicolò Caporale
Postdoctoral Associate -
 Davide Castaldi
Davide Castaldi
Postdoc Fellow -
 Lorenza Culotta
Lorenza Culotta
Senior Technician -
 Francesco Elli
Francesco Elli
Affiliate Scientist -
 Marlene Cristina Faria Pereira
Marlene Cristina Faria Pereira
Affiliate Scientist -
 Samuele Galimi
Samuele Galimi
Postgraduate Fellow -
 Oliviero Leonardi
Oliviero Leonardi
PhD Student -
 Lisa Mainardi
Lisa Mainardi
Scientific Visitor -
 Alessandro Melon
Alessandro Melon
Undergraduate Intern -
 Riccardo Nagni
Riccardo Nagni
Undergraduate Intern -
 Marco Tullio Rigoli
Marco Tullio Rigoli
Affiliate Scientist -
 Ludovico Rizzuti
Ludovico Rizzuti
Affiliate Scientist -
 Adrianos Skaros
Adrianos Skaros
Affiliate Scientist -
 Sarah Stucchi
Sarah Stucchi
PhD Student -
 Alejandro Lopez Tobon
Alejandro Lopez Tobon
Scientific Advisor -
 Alessandro Valente
Alessandro Valente
Affiliate Scientist -
 Alessia Valenti
Alessia Valenti
PhD Student -
 Emanuele Villa
Emanuele Villa
Senior Staff Scientist -
 Alessandro Vitriolo
Alessandro Vitriolo
Affiliate Scientist -
 Narges Yahyazadeh
Narges Yahyazadeh
Scientific Visitor
Publications
-
04/2022 - BioRxiv
Benchmarking brain organoid recapitulation of fetal corticogenesis
Brain organoids are becoming increasingly relevant to dissect the molecular mechanisms underlying psychiatric and neurological conditions. The in vitro recapitulation of key features of human brain development affords the unique opportunity of investigating the developmental antecedents of neuropsychiatric conditions in the context of the actual patients’ genetic backgrounds. Specifically, multiple strategies of brain organoid (BO) differentiation have […]
-
04/2022 - Cell Reports
CHD8 haploinsufficiency links autism to transient alterations in excitatory and inhibitory trajectories
Mutations in the chromodomain helicase DNA-binding 8 (CHD8) gene are a frequent cause of autism spectrum disorder (ASD). While its phenotypic spectrum often encompasses macrocephaly, implicating cortical abnormalities, how CHD8 haploinsufficiency affects neurodevelopmental is unclear. Here, employing human cerebral organoids, we find that CHD8 haploinsufficiency disrupted neurodevelopmental trajectories with an accelerated and delayed generation of, respectively, inhibitory and excitatory neurons that yields, at days 60 […]
-
03/2022 - International Journal of Molecular Sciences
The ENDpoiNTs Project: Novel Testing Strategies for Endocrine Disruptors Linked to Developmental Neurotoxicity
Ubiquitous exposure to endocrine-disrupting chemicals (EDCs) has caused serious concerns about the ability of these chemicals to affect neurodevelopment, among others. Since endocrine disruption (ED)-induced developmental neurotoxicity (DNT) is hardly covered by the chemical testing tools that are currently in regulatory use, the Horizon 2020 research and innovation action ENDpoiNTs has been launched to fill […]
-
10/2021 - Frontiers in Cellular Neuroscience
Novel in vitro Experimental Approaches to Study Myelination and Remyelination in the Central Nervous System
Myelin is the lipidic insulating structure enwrapping axons and allowing fast saltatory nerve conduction. In the central nervous system, myelin sheath is the result of the complex packaging of multilamellar extensions of oligodendrocyte (OL) membranes. Before reaching myelinating capabilities, OLs undergo a very precise program of differentiation and maturation that starts from OL precursor cells […]