Testa Group
The Testa Group harnesses the unprecedented potential of cell reprogramming to study the molecular basis of human neuropsychiatric and neurological diseases (NPD), by chasing the dynamics of their unfolding in physio pathologically relevant models and straddling multiple scales of analysis from single cell resolution to organismal function.
One of the most tangible outputs of somatic cell reprogramming has been a paradigm shift in our ability to model human diseases, for which fundamental limitations have been so far: i) the scarce availability of primary diseased tissues, which is particularly salient for disorders of the nervous system; and ii) the difficulty of reconstructing developmental and patient-specific trajectories during the unfolding of diseases.
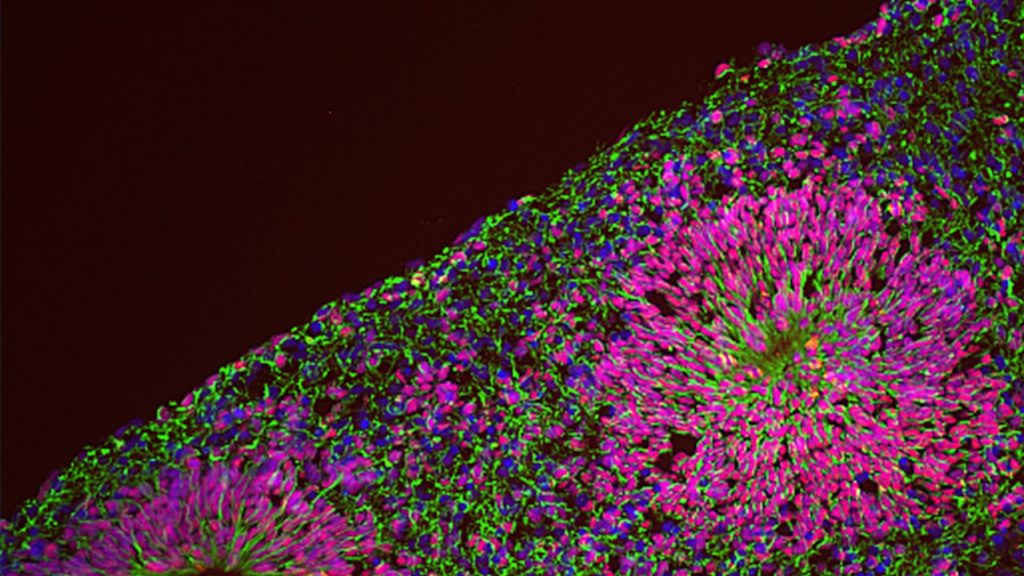
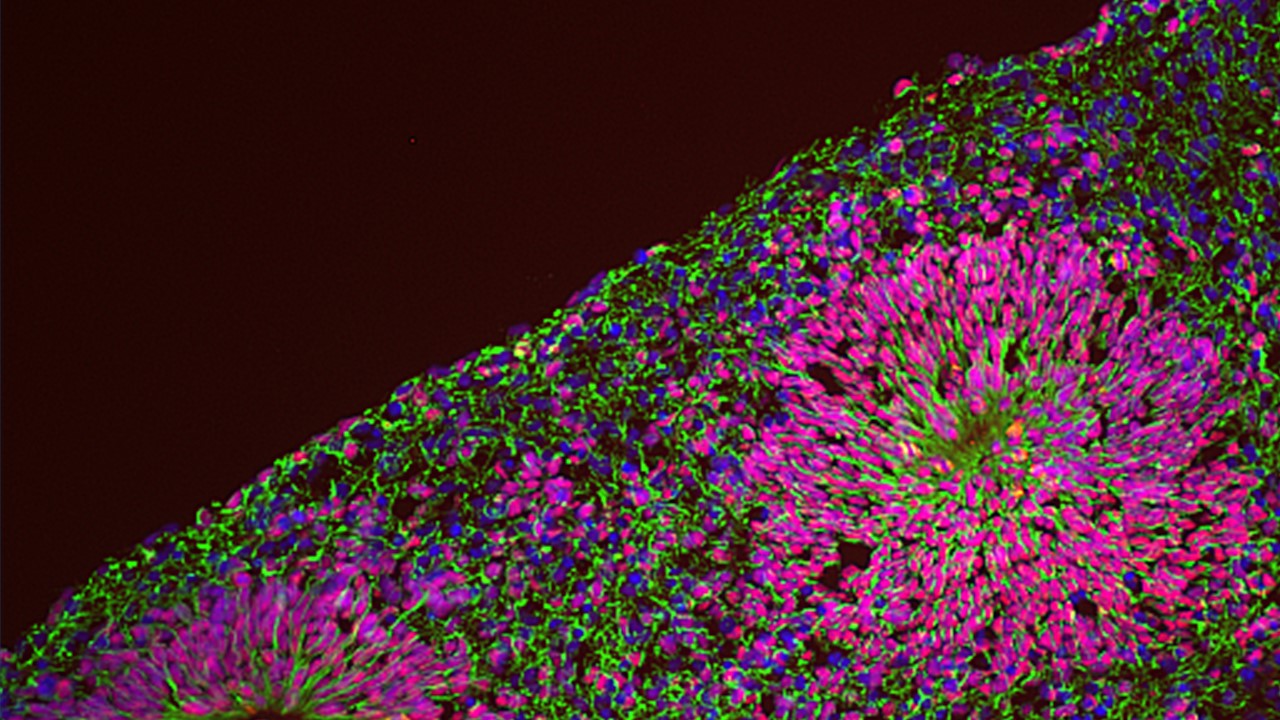
We are thus pursuing NPD modelling with human induced pluripotent stem cells (iPSC) coupled with the differentiation into relevant lineages through a range of complementary experimental paradigms, including glutamatergic neurons by induced expression of neurogenin-2 (NGN2), neural crest stem cells and 3-dimensional brain organoids that recapitulate salient stages of early brain development, including the diversity of cell populations unique to the human brain. This is allowing us to dissect the genetic and environmental components of NPD pathogenesis, by means of several “omics” approaches at a bulk and single cell resolution integrated with high throughput imaging and functional in vitro and in vivo assays.
The Testa Group focuses on a uniquely informative range of syndromes featuring intellectual disability and autism spectrum disorder that are caused by mutations or dosage alterations in epigenetic regulators and transcription factors, including Williams-Beuren Syndrome and 7q11.23 microduplication Syndrome, Kabuki Syndrome, ADNP-related autistic spectrum disorders, Weaver Syndrome, Gabriele-Testa-DeVries Syndrome, as well as on paradigmatic environmental factors that adversely impact on neurodevelopment, namely endocrine disruptive chemicals.
Finally, the prism of human neurodevelopmental disorders is also allowing us to investigate the logic of the gene regulatory networks underlying the evolution of the modern human face and brain, integrating the analysis of craniofacial dysmorphologies and brain alterations to illuminate the evolutionary-developmental trajectories underlying the modern human condition.
Testa Group 2023

Group members
-
 Giuseppe Testa
Giuseppe Testa
Head of Neurogenomics -
 Davide Aprile
Davide Aprile
Postdoc -
 Lorenzo Basile
Lorenzo Basile
Undergraduate Intern -
 Nicolò Caporale
Nicolò Caporale
Postdoctoral Associate -
 Davide Castaldi
Davide Castaldi
Postdoc Fellow -
 Lorenza Culotta
Lorenza Culotta
Senior Technician -
 Francesco Elli
Francesco Elli
Affiliate Scientist -
 Marlene Cristina Faria Pereira
Marlene Cristina Faria Pereira
Affiliate Scientist -
 Samuele Galimi
Samuele Galimi
Postgraduate Fellow -
 Oliviero Leonardi
Oliviero Leonardi
PhD Student -
 Lisa Mainardi
Lisa Mainardi
Scientific Visitor -
 Alessandro Melon
Alessandro Melon
Undergraduate Intern -
 Riccardo Nagni
Riccardo Nagni
Undergraduate Intern -
 Marco Tullio Rigoli
Marco Tullio Rigoli
Affiliate Scientist -
 Ludovico Rizzuti
Ludovico Rizzuti
Affiliate Scientist -
 Adrianos Skaros
Adrianos Skaros
Affiliate Scientist -
 Sarah Stucchi
Sarah Stucchi
PhD Student -
 Alejandro Lopez Tobon
Alejandro Lopez Tobon
Scientific Advisor -
 Alessandro Valente
Alessandro Valente
Affiliate Scientist -
 Alessia Valenti
Alessia Valenti
PhD Student -
 Emanuele Villa
Emanuele Villa
Senior Staff Scientist -
 Alessandro Vitriolo
Alessandro Vitriolo
Affiliate Scientist -
 Narges Yahyazadeh
Narges Yahyazadeh
Scientific Visitor
Publications
-
06/2020 - Molecular Autism
The sociability spectrum: evidence from reciprocal genetic copy number variations
Sociability entails some of the most complex behaviors processed by the central nervous system. It includes the detection, integration, and interpretation of social cues and elaboration of context-specific responses that are quintessentially species-specific. There is an ever-growing accumulation of molecular associations to autism spectrum disorders (ASD), from causative genes to endophenotypes across multiple functional layers; […]
-
06/2020 - Neurosci Insights
KMT2B and Neuronal Transdifferentiation: Bridging Basic Chromatin Mechanisms to Disease Actionability
The role of bona fide epigenetic regulators in the process of neuronal transdifferentiation was until recently largely uncharacterized, despite their key role in the physiological processes of neural fate acquisition and maintenance. In this commentary, we describe the main findings of our recent paper “KMT2B is selectively required for neuronal transdifferentiation, and its loss exposes dystonia candidate […]
-
06/2020 - Molecular Autism
Copy number variants (CNVs): a powerful tool for iPSC-based modelling of ASD
Patients diagnosed with chromosome microdeletions or duplications, known as copy number variants (CNVs), present a unique opportunity to investigate the relationship between patient genotype and cell phenotype. CNVs have high genetic penetrance and give a good correlation between gene locus and patient clinical phenotype. This is especially effective for the study of patients with neurodevelopmental […]
-
04/2020 - Clinical Genetics
A small 7q11.23 microduplication involving GTF2I in a family with intellectual disability