Vannini Group
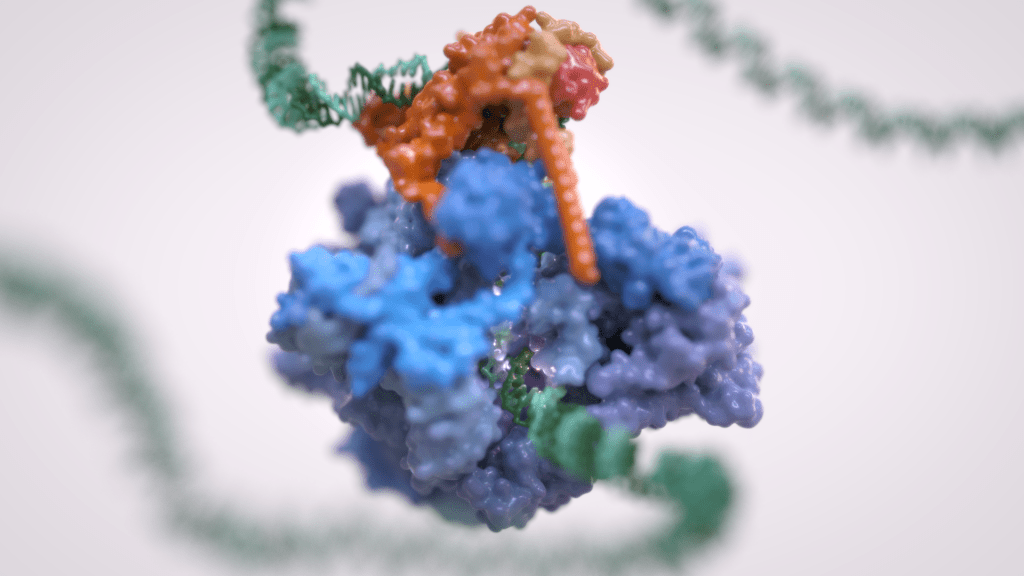
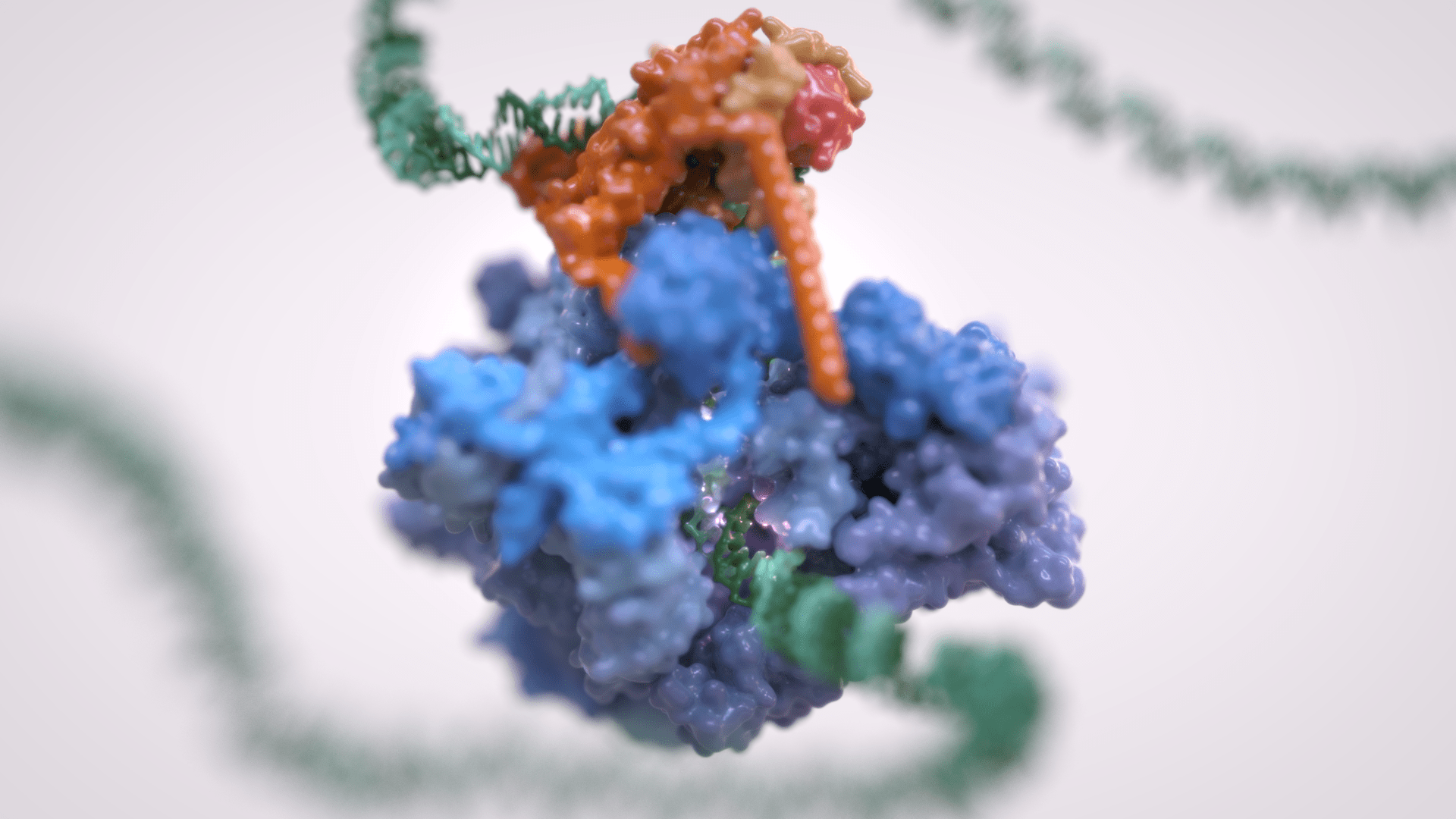
Gene transcription is the first step that controls the expression of the genetic information encoded in a genome and ultimately underlies cell differentiation and organism development. Eukaryotic gene transcription occurs in the context of highly structured and organised genomes and acts as a coordinator of numerous events co-occurring in the nucleus. Eukaryotic transcription relies on three different RNA polymerases: RNA polymerase I (Pol I) transcribes ribosomal RNA, RNA polymerase II (Pol II) synthesizes messenger RNAs and RNA polymerase III (Pol III) produces short and non-translated RNAs, including the entire pool of tRNAs, which are essential for cell growth.
For a long time, it was assumed that only Pol II was regulated whereas Pol I and Pol III did not require such control. However, it is now clear that RNA polymerase III transcription is tightly regulated and a determinant of organismal growth. Pol III deregulation is observed in many forms of cancer and Pol III genetic mutations cause severe neurodegenerative diseases.
Furthermore, Pol III and its associated factors play a paramount role into genome structure and organisation. These “extra-transcriptional roles” are carried out throughout interactions with other cellular components such as retroelement transposition machineries, Structural Maintenance of Chromosome (SMC) complexes and specific chromatin remodellers.
The Vannini Group employs an Integrative Structural Biology approach, combining cutting-edge cryo-EM analysis, x-ray diffraction data, cross-linking and native mass-spectrometry. We integrate the structural data with molecular and cellular biology techniques in order to obtain a comprehensive view of these fundamental processes and how their mis-regulation can lead to cancer and neurodegenerative diseases.
Group members
-
 Alessandro Vannini
Alessandro Vannini
Head of Structural Biology Research Centre -
 Alessandro Borsellini
Alessandro Borsellini
Postdoc -
 Giacomo Ettore Casale
Giacomo Ettore Casale
Postdoc -
 Valentina Cecatiello
Valentina Cecatiello
Senior Technician -
 Sebastian Chamera
Sebastian Chamera
Postdoc -
 Fabiola Iommazzo
Fabiola Iommazzo
PhD Student -
 Thomas Noé Perry
Thomas Noé Perry
Postdoc -
 Fabio Pessina
Fabio Pessina
Senior Technician -
 Mariavittoria Pizzinga
Mariavittoria Pizzinga
Postdoc -
 Ewan Ramsay
Ewan Ramsay
Senior Staff Scientist -
 Ankit Roy
Ankit Roy
PhD Student -
 Syed Zawar Shah
Syed Zawar Shah
PhD Student
Publications
-
07/2020 - Nature Metabolism
A micronutrient with major effects on cancer cell viability
Selenium is a micronutrient essential for the generation of selenoproteins, which function predominantly by detoxifying cellular reactive oxygen species. In this issue, Carlisle et al. describe a novel mechanism whereby perturbing selenium utilization via inhibition of SEPHS2, a component of the selenocysteine-biosynthesis pathway, results in selenide poisoning and cancer cell death.
-
07/2020 - Molecular Cell
Human Condensin I and II Drive Extensive ATP-Dependent Compaction of Nucleosome-Bound DNA
Structural maintenance of chromosomes (SMC) complexes are essential for genome organization from bacteria to humans, but their mechanisms of action remain poorly understood. Here, we characterize human SMC complexes condensin I and II and unveil the architecture of the human condensin II complex, revealing two putative DNA-entrapment sites. Using single-molecule imaging, we demonstrate that both […]
-
06/2020 - Cell
Hybrid Gene Origination Creates Human-Virus Chimeric Proteins during Infection
RNA viruses are a major human health threat. The life cycles of many highly pathogenic RNA viruses like influenza A virus (IAV) and Lassa virus depends on host mRNA, because viral polymerases cleave 5′-m7G-capped host transcripts to prime viral mRNA synthesis (“cap-snatching”). We hypothesized that start codons within cap-snatched host transcripts could generate chimeric human-viral […]
-
06/2020 - Nature Communications
DNA origami-based single-molecule force spectroscopy elucidates RNA Polymerase III pre-initiation complex stability
The TATA-binding protein (TBP) and a transcription factor (TF) IIB-like factor are important constituents of all eukaryotic initiation complexes. The reason for the emergence and strict requirement of the additional initiation factor Bdp1 in the RNA polymerase (RNAP) III system, however, remained elusive. A poorly studied aspect in this context is the effect of DNA […]
-
11/2019 - Molecular Cell
TFIIIC Binding to Alu Elements Controls Gene Expression via Chromatin Looping and Histone Acetylation
How repetitive elements, epigenetic modifications, and architectural proteins interact ensuring proper genome expression remains poorly understood. Here, we report regulatory mechanisms unveiling a central role of Alu elements (AEs) and RNA polymerase III transcription factor C (TFIIIC) in structurally and functionally modulating the genome via chromatin looping and histone acetylation. Upon serum deprivation, a subset […]