Vannini Group
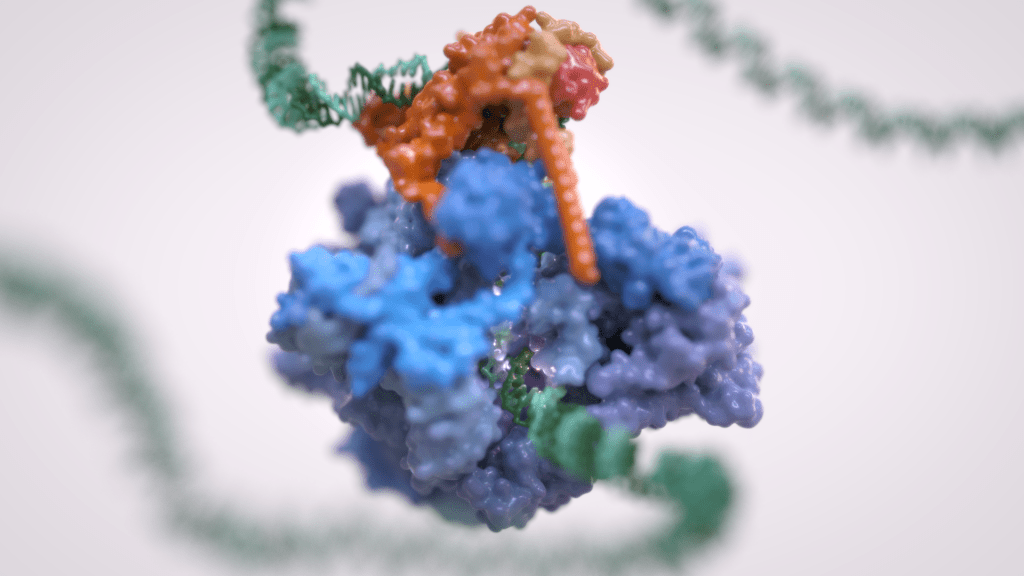
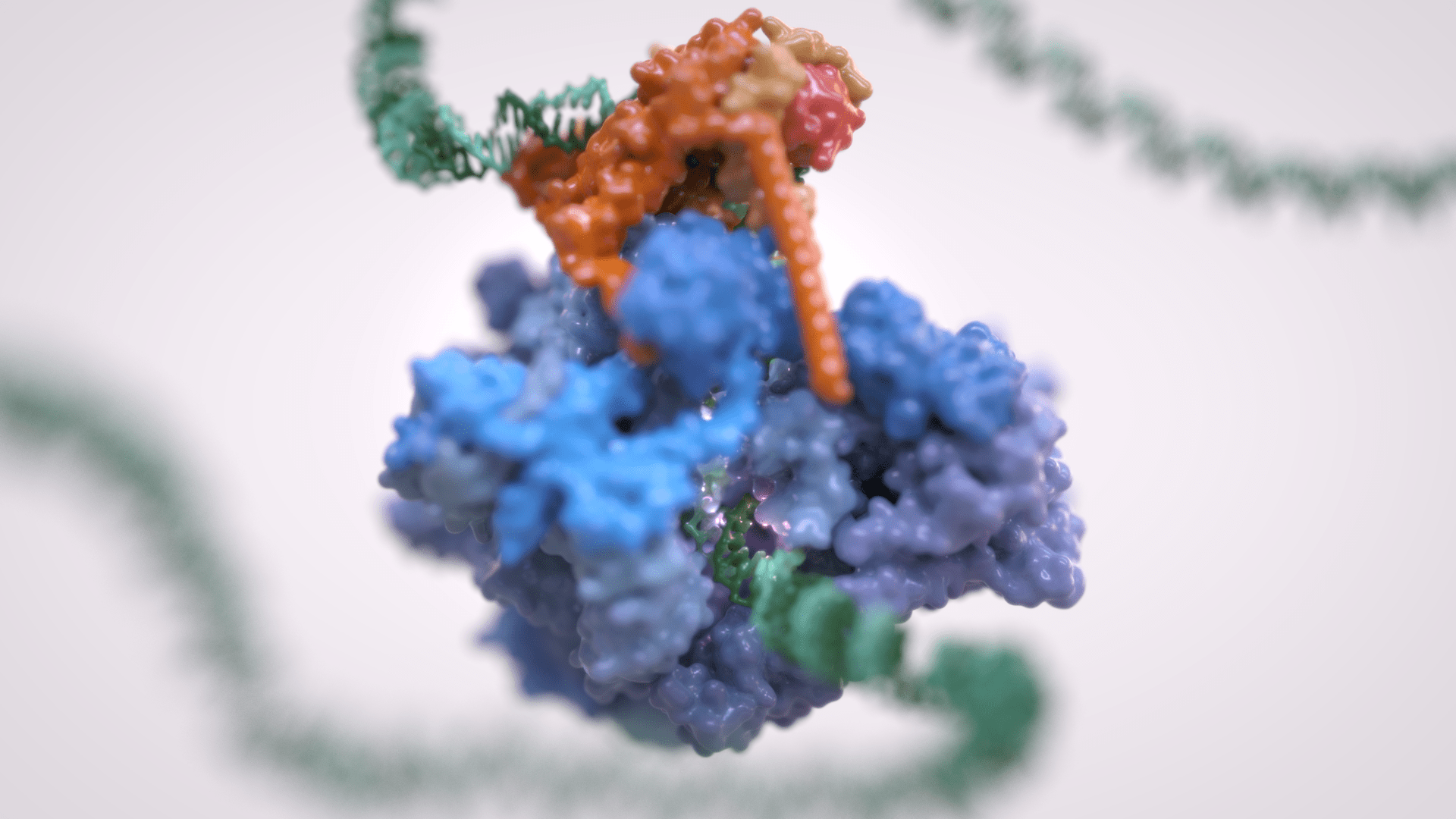
Gene transcription is the first step that controls the expression of the genetic information encoded in a genome and ultimately underlies cell differentiation and organism development. Eukaryotic gene transcription occurs in the context of highly structured and organised genomes and acts as a coordinator of numerous events co-occurring in the nucleus. Eukaryotic transcription relies on three different RNA polymerases: RNA polymerase I (Pol I) transcribes ribosomal RNA, RNA polymerase II (Pol II) synthesizes messenger RNAs and RNA polymerase III (Pol III) produces short and non-translated RNAs, including the entire pool of tRNAs, which are essential for cell growth.
For a long time, it was assumed that only Pol II was regulated whereas Pol I and Pol III did not require such control. However, it is now clear that RNA polymerase III transcription is tightly regulated and a determinant of organismal growth. Pol III deregulation is observed in many forms of cancer and Pol III genetic mutations cause severe neurodegenerative diseases.
Furthermore, Pol III and its associated factors play a paramount role into genome structure and organisation. These “extra-transcriptional roles” are carried out throughout interactions with other cellular components such as retroelement transposition machineries, Structural Maintenance of Chromosome (SMC) complexes and specific chromatin remodellers.
The Vannini Group employs an Integrative Structural Biology approach, combining cutting-edge cryo-EM analysis, x-ray diffraction data, cross-linking and native mass-spectrometry. We integrate the structural data with molecular and cellular biology techniques in order to obtain a comprehensive view of these fundamental processes and how their mis-regulation can lead to cancer and neurodegenerative diseases.
Group members
-
 Alessandro Vannini
Alessandro Vannini
Head of Structural Biology Research Centre -
 Alessandro Borsellini
Alessandro Borsellini
Postdoc -
 Giacomo Ettore Casale
Giacomo Ettore Casale
Postdoc -
 Valentina Cecatiello
Valentina Cecatiello
Senior Technician -
 Sebastian Chamera
Sebastian Chamera
Postdoc -
 Fabiola Iommazzo
Fabiola Iommazzo
PhD Student -
 Thomas Noé Perry
Thomas Noé Perry
Postdoc -
 Fabio Pessina
Fabio Pessina
Senior Technician -
 Mariavittoria Pizzinga
Mariavittoria Pizzinga
Postdoc -
 Ewan Ramsay
Ewan Ramsay
Senior Staff Scientist -
 Ankit Roy
Ankit Roy
PhD Student -
 Syed Zawar Shah
Syed Zawar Shah
PhD Student
Publications
-
05/2008 - Analytical Chemistry
Multiplexed proteomics mapping of yeast RNA polymerase II and III allows near-complete sequence coverage and reveals several novel phosphorylation sites
The multisubunit RNA polymerases (Pols) II and III synthesize mainly eukaryotic mRNAs and tRNAs, respectively. Pol II and Pol III are protein complexes consisting of 12 and 17 subunits. Here we analyzed both yeast Pol II and Pol III by multiplexed mass spectrometric analysis using various proteases and both collision induced and electron transfer dissociation. […]
-
01/2008 - Annu Rev Biophys
Structure of eukaryotic RNA polymerases
The eukaryotic RNA polymerases Pol I, Pol II, and Pol III are the central multiprotein machines that synthesize ribosomal, messenger, and transfer RNA, respectively. Here we provide a catalog of available structural information for these three enzymes. Most structural data have been accumulated for Pol II and its functional complexes. These studies have provided insights […]
-
10/2007 - Structure
Structural biology of RNA polymerase III: mass spectrometry elucidates subcomplex architecture
RNA polymerases (Pol) II and III synthesize eukaryotic mRNAs and tRNAs, respectively. The crystal structure of the 12 subunit Pol II is known, but only limited structural information is available for the 17 subunit Pol III. Using mass spectrometry (MS), we correlated masses of Pol II complexes with the Pol II structure. Analysis of Pol […]
-
09/2007 - EMBO Rep
Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8-substrate complex
Histone deacetylases (HDACs)-an enzyme family that deacetylates histones and non-histone proteins-are implicated in human diseases such as cancer, and the first-generation of HDAC inhibitors are now in clinical trials. Here, we report the 2.0 A resolution crystal structure of a catalytically inactive HDAC8 active-site mutant, Tyr306Phe, bound to an acetylated peptidic substrate. The structure clarifies […]
-
10/2004 - Proc Natl Acad Sci U S A
Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor
Histone deacetylases (HDACs) are a family of enzymes involved in the regulation of gene expression, DNA repair, and stress response. These processes often are altered in tumors, and HDAC inhibitors have had pronounced antitumor activity with promising results in clinical trials. Here, we report the crystal structure of human HDAC8 in complex with a hydroxamic […]